The influence of sensitization on mechanisms of organophosphorus pesticide-induced airway hyperreactivity
- PMID: 25897622
- PMCID: PMC4742952
- DOI: 10.1165/rcmb.2014-0444OC
The influence of sensitization on mechanisms of organophosphorus pesticide-induced airway hyperreactivity
Abstract
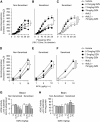
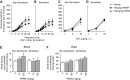
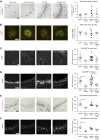
We previously demonstrated that antigen sensitization increases vulnerability to airway hyperreactivity induced by the organophosphorus pesticide (OP) parathion. Sensitization also changes the mechanism of parathion-induced airway hyperreactivity to one that is dependent on IL-5. To determine whether this effect can be generalized to other OPs, and to other classes of pesticides, we measured airway responsiveness to vagal stimulation or intravenous acetylcholine in nonsensitized and ovalbumin-sensitized guinea pigs 24 hours after a single subcutaneous injection of the OPs diazinon or chlorpyrifos, or the pyrethroid permethrin. Sensitization exacerbated the effects of chlorpyrifos on bronchoconstriction in response to vagal stimulation or intravenous acetylcholine. Pretreatment with function-blocking IL-5 antibody prevented chlorpyrifos-induced airway hyperreactivity in sensitized, but not in nonsensitized, guinea pigs. In sensitized guinea pigs, blocking IL-5 decreased eosinophil activation, as measured by decreased eosinophil major basic protein in the trachea. In contrast, sensitization did not alter diazinon-induced airway hyperreactivity, and permethrin did not cause airway hyperreactivity in either nonsensitized or sensitized guinea pigs. None of the pesticides affected inflammatory cells in the bronchoalveolar lavage fluid or blood. We have previously shown that three different OPs cause airway hyperreactivity via loss of neuronal M2 muscarinic receptor function. Similar to parathion, but unlike diazinon, the mechanism of chlorpyrifos-induced airway hyperreactivity is changed by sensitization. Thus, OP-induced airway hyperreactivity is dependent on sensitization status and on the OP used, which may influence therapeutic approaches.
Keywords: airway hyperreactivity; eosinophils; organophosphorus pesticides; permethrin; sensitization.
Figures

References
-
- Gallo MA, Lawryk NJ. Organic phosphorus pesticides. In: Hayes WJ, Laws ER, editors. New York: Academic Press, Inc.; 1991. pp. 917–972. Handbook of pesticide toxicology.
-
- Barr DB, Wong LY, Bravo R, Weerasekera G, Odetokun M, Restrepo P, Kim DG, Fernandez C, Whitehead RD, Jr, Perez J, et al. Urinary concentrations of dialkylphosphate metabolites of organophosphorus pesticides: National Health and Nutrition Examination Survey 1999–2004. Int J Environ Res Public Health. 2011;8:3063–3098. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES014521/ES/NIEHS NIH HHS/United States
- R01 ES017592/ES/NIEHS NIH HHS/United States
- R01 HL124165/HL/NHLBI NIH HHS/United States
- R01 ES014601/ES/NIEHS NIH HHS/United States
- F32 ES014521/ES/NIEHS NIH HHS/United States
- HL124165/HL/NHLBI NIH HHS/United States
- ES017592/ES/NIEHS NIH HHS/United States
- R01 HL131525/HL/NHLBI NIH HHS/United States
- HL113023/HL/NHLBI NIH HHS/United States
- R01 HL113023/HL/NHLBI NIH HHS/United States
- T32 HL083808/HL/NHLBI NIH HHS/United States
- AR061567/AR/NIAMS NIH HHS/United States
- ES014601/ES/NIEHS NIH HHS/United States
- R01 AR061567/AR/NIAMS NIH HHS/United States

