Adding a treatment arm to an ongoing clinical trial: a review of methodology and practice
- PMID: 25897686
- PMCID: PMC4457999
- DOI: 10.1186/s13063-015-0697-y
Adding a treatment arm to an ongoing clinical trial: a review of methodology and practice
Abstract
Incorporating an emerging therapy as a new randomisation arm in a clinical trial that is open to recruitment would be desirable to researchers, regulators and patients to ensure that the trial remains current, new treatments are evaluated as quickly as possible, and the time and cost for determining optimal therapies is minimised. It may take many years to run a clinical trial from concept to reporting within a rapidly changing drug development environment; hence, in order for trials to be most useful to inform policy and practice, it is advantageous for them to be able to adapt to emerging therapeutic developments. This paper reports a comprehensive literature review on methodologies for, and practical examples of, amending an ongoing clinical trial by adding a new treatment arm. Relevant methodological literature describing statistical considerations required when making this specific type of amendment is identified, and the key statistical concepts when planning the addition of a new treatment arm are extracted, assessed and summarised. For completeness, this includes an assessment of statistical recommendations within general adaptive design guidance documents. Examples of confirmatory ongoing trials designed within the frequentist framework that have added an arm in practice are reported; and the details of the amendment are reviewed. An assessment is made as to how well the relevant statistical considerations were addressed in practice, and the related implications. The literature review confirmed that there is currently no clear methodological guidance on this topic, but that guidance would be advantageous to help this efficient design amendment to be used more frequently and appropriately in practice. Eight confirmatory trials were identified to have added a treatment arm, suggesting that trials can benefit from this amendment and that it can be practically feasible; however, the trials were not always able to address the key statistical considerations, often leading to uninterpretable or invalid outcomes. If the statistical concepts identified within this review are considered and addressed during the design of a trial amendment, it is possible to effectively assess a new treatment arm within an ongoing trial without compromising the original trial outcomes.
Figures
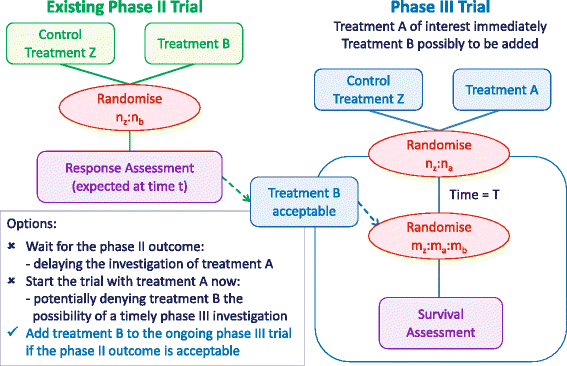

References
-
- CRUK. Cancer Survival for Common Cancers: Trends over Time. 2014. http://www.cancerresearchuk.org/cancer-info/cancerstats/survival/common-.... Accessed 29 Jan 2015.
-
- Pong A, Chow SC. Handbook of Adaptive Designs in Pharmaceutical and Clinical Development. New York: Taylor & Francis; 2010.
-
- FDA. Draft Guidance on Adaptive Design Clinical Trials for Drugs and Biologics. Rockville, MD. 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed 29 Jan 2015.
-
- CHMP (Committee for Medicinal Products for Human Use) Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design. London: EMEA (European Medicines Agency); 2007.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

