Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus
- PMID: 25897961
- PMCID: PMC4405337
- DOI: 10.1371/journal.pone.0124595
Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus
Abstract
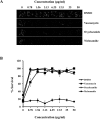
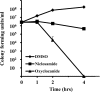
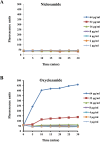
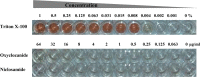
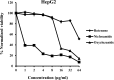
Staphylococcus aureus is a Gram-positive bacterium that has become the leading cause of hospital acquired infections in the US. Repurposing Food and Drug Administration (FDA) approved drugs for antimicrobial therapy involves lower risks and costs compared to de novo development of novel antimicrobial agents. In this study, we examined the antimicrobial properties of two commercially available anthelmintic drugs. The FDA approved drug niclosamide and the veterinary drug oxyclozanide displayed strong in vivo and in vitro activity against methicillin resistant S. aureus (minimum inhibitory concentration (MIC): 0.125 and 0.5 μg/ml respectively; minimum effective concentration: ≤ 0.78 μg/ml for both drugs). The two drugs were also effective against another Gram-positive bacteria Enterococcus faecium (MIC 0.25 and 2 μg/ml respectively), but not against the Gram-negative species Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter aerogenes. The in vitro antimicrobial activity of niclosamide and oxyclozanide were determined against methicillin, vancomycin, linezolid or daptomycin resistant S. aureus clinical isolates, with MICs at 0.0625-0.5 and 0.125-2 μg/ml for niclosamide and oxyclozanide respectively. A time-kill study demonstrated that niclosamide is bacteriostatic, whereas oxyclozanide is bactericidal. Interestingly, oxyclozanide permeabilized the bacterial membrane but neither of the anthelmintic drugs exhibited demonstrable toxicity to sheep erythrocytes. Oxyclozanide was non-toxic to HepG2 human liver carcinoma cells within the range of its in vitro MICs but niclosamide displayed toxicity even at low concentrations. These data show that the salicylanilide anthelmintic drugs niclosamide and oxyclozanide are suitable candidates for mechanism of action studies and further clinical evaluation for treatment of staphylococcal infections.
Conflict of interest statement
Figures

Similar articles
-
The in vitro antibacterial activity of the anthelmintic drug oxyclozanide against common small animal bacterial pathogens.Vet Dermatol. 2019 Aug;30(4):314-e87. doi: 10.1111/vde.12755. Epub 2019 May 7. Vet Dermatol. 2019. PMID: 31062461
-
Synergistic combinations of anthelmintic salicylanilides oxyclozanide, rafoxanide, and closantel with colistin eradicates multidrug-resistant colistin-resistant Gram-negative bacilli.J Antibiot (Tokyo). 2019 Aug;72(8):605-616. doi: 10.1038/s41429-019-0186-8. Epub 2019 Apr 26. J Antibiot (Tokyo). 2019. PMID: 31028351
-
The Anthelmintic Drug Niclosamide Synergizes with Colistin and Reverses Colistin Resistance in Gram-Negative Bacilli.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e02574-18. doi: 10.1128/AAC.02574-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30917988 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus.Clin Microbiol Infect. 2007 Jun;13 Suppl 2:17-24. doi: 10.1111/j.1469-0691.2007.01722.x. Clin Microbiol Infect. 2007. PMID: 17488372 Review.
Cited by
-
Characterization of Five Novel Anti-MRSA Compounds Identified Using a Whole-Animal Caenorhabditis elegans/Galleria mellonella Sequential-Screening Approach.Antibiotics (Basel). 2020 Jul 27;9(8):449. doi: 10.3390/antibiotics9080449. Antibiotics (Basel). 2020. PMID: 32726955 Free PMC article.
-
The ionophore oxyclozanide enhances tobramycin killing of Pseudomonas aeruginosa biofilms by permeabilizing cells and depolarizing the membrane potential.J Antimicrob Chemother. 2019 Apr 1;74(4):894-906. doi: 10.1093/jac/dky545. J Antimicrob Chemother. 2019. PMID: 30624737 Free PMC article.
-
Implantable antimicrobial biomaterials for local drug delivery in bone infection models.Acta Biomater. 2019 Jul 15;93:2-11. doi: 10.1016/j.actbio.2019.01.015. Epub 2019 Jan 14. Acta Biomater. 2019. PMID: 30654212 Free PMC article. Review.
-
An FDA-Drug Library Screen for Compounds with Bioactivities against Meticillin-Resistant Staphylococcus aureus (MRSA).Antibiotics (Basel). 2015 Oct 9;4(4):424-34. doi: 10.3390/antibiotics4040424. Antibiotics (Basel). 2015. PMID: 27025633 Free PMC article.
-
In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria.Antibiotics (Basel). 2022 Feb 22;11(3):291. doi: 10.3390/antibiotics11030291. Antibiotics (Basel). 2022. PMID: 35326755 Free PMC article.
References
-
- Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46 Suppl 5:S344–9. - PubMed
-
- Jones RN. Global epidemiology of antimicrobial resistance among community-acquired and nosocomial pathogens: a five-year summary from the SENTRY Antimicrobial Surveillance Program (1997–2001). Seminars in respiratory and critical care medicine. 2003;24(1):121–34. - PubMed
-
- Gottlieb GS, Fowler VG Jr., Kong LK, McClelland RS, Gopal AK, Marr KA, et al. Staphylococcus aureus bacteremia in the surgical patient: a prospective analysis of 73 postoperative patients who developed Staphylococcus aureus bacteremia at a tertiary care facility. Journal of the American College of Surgeons. 2000;190(1):50–7. - PubMed
-
- Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2007;13(3):257–63. - PubMed
-
- Feng Y, Chen CJ, Su LH, Hu S, Yu J, Chiu CH. Evolution and pathogenesis of Staphylococcus aureus: lessons learned from genotyping and comparative genomics. FEMS microbiology reviews. 2008;32(1):23–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

