RTA 408, A Novel Synthetic Triterpenoid with Broad Anticancer and Anti-Inflammatory Activity
- PMID: 25897966
- PMCID: PMC4405374
- DOI: 10.1371/journal.pone.0122942
RTA 408, A Novel Synthetic Triterpenoid with Broad Anticancer and Anti-Inflammatory Activity
Abstract
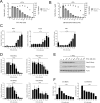
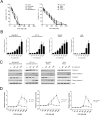
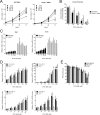
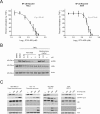
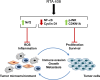
Semi-synthetic triterpenoids are antioxidant inflammation modulator (AIM) compounds that inhibit tumor cell growth and metastasis. Compounds in the AIM class bind to Keap1 and attenuate Nrf2 degradation. In the nucleus, Nrf2 increases antioxidant gene expression and reduces pro-inflammatory gene expression. By increasing Nrf2 activity, AIMs reduce reactive oxygen species and inflammation in the tumor microenvironment, which reverses tumor-mediated immune evasion and inhibits tumor growth and metastasis. AIMs also directly inhibit tumor cell growth by modulating oncogenic signaling pathways, such as IKKβ/NF-κB. Here, we characterized the in vitro antioxidant, anti-inflammatory, and anticancer activities of RTA 408, a novel AIM that is currently being evaluated in patients with advanced malignancies. At low concentrations (≤ 25 nM), RTA 408 activated Nrf2 and suppressed nitric oxide and pro-inflammatory cytokine levels in interferon-γ-stimulated RAW 264.7 macrophage cells. At higher concentrations, RTA 408 inhibited tumor cell growth (GI50 = 260 ± 74 nM) and increased caspase activity in tumor cell lines, but not in normal primary human cells. Consistent with the direct effect of AIMs on IKKβ, RTA 408 inhibited NF-κB signaling and decreased cyclin D1 levels at the same concentrations that inhibited cell growth and induced apoptosis. RTA 408 also increased CDKN1A (p21) levels and JNK phosphorylation. The in vitro activity profile of RTA 408 is similar to that of bardoxolone methyl, which was well-tolerated by patients at doses that demonstrated target engagement. Taken together, these data support clinical evaluation of RTA 408 for cancer treatment.
Conflict of interest statement
Figures
References
-
- Liu J. Oleanolic acid and ursolic acid: research perspectives. J Ethnopharmacol 2005. August 22;100(1–2):92–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

