GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane
- PMID: 25897971
- PMCID: PMC4430820
- DOI: 10.1038/ncomms7969
GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane
Abstract
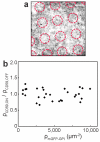
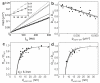
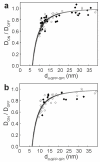
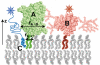
The organization of proteins and lipids in the plasma membrane has been the subject of a long-lasting debate. Membrane rafts of higher lipid chain order were proposed to mediate protein interactions, but have thus far not been directly observed. Here we use protein micropatterning combined with single-molecule tracking to put current models to the test: we rearranged lipid-anchored raft proteins (glycosylphosphatidylinositol(GPI)-anchored-mGFP) directly in the live cell plasma membrane and measured the effect on the local membrane environment. Intriguingly, this treatment does neither nucleate the formation of an ordered membrane phase nor result in any enrichment of nanoscopic-ordered domains within the micropatterned regions. In contrast, we find that immobilized mGFP-GPIs behave as inert obstacles to the diffusion of other membrane constituents without influencing their membrane environment over distances beyond their physical size. Our results indicate that phase partitioning is not a fundamental element of protein organization in the plasma membrane.
Figures


References
-
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–31. - PubMed
-
- Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. - PubMed
-
- Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochimica Et Biophysica Acta-Biomembranes. 2004;1666:2–18. - PubMed
-
- Niemela PS, et al. Membrane proteins diffuse as dynamic complexes with lipids. J Am Chem Soc. 2010;132:7574–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

