The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo
- PMID: 25898005
- PMCID: PMC4428110
- DOI: 10.7554/eLife.06670
The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo
Abstract
Effective mucosal adjuvants enhance the magnitude and quality of the vaccine response. Cyclic di-GMP (CDG) is a promising mucosal vaccine adjuvant. However, its in vivo mechanisms are unclear. Here, we showed, in mice, that CDG elicits stronger Ab and TH responses than the mammalian 2'3'-cyclic GMP-AMP (cGAMP), and generated better protection against Streptococcus pneumoniae infection than 2'3'-cGAMP adjuvanted vaccine. We identified two in vivo mechanisms of CDG. First, intranasally administered CDG greatly enhances Ag uptake, including pinocytosis and receptor-mediated endocytosis in vivo. The enhancement depends on MPYS (STING, MITA) expression in CD11C(+) cells. Second, we found that CDG selectively activated pinocytosis-efficient-DCs, leading to T(H) polarizing cytokines IL-12p70, IFNγ, IL-5, IL-13, IL-23, and IL-6 production in vivo. Notably, CDG induces IFNλ, but not IFNβ, in vivo. Our study revealed previously unrecognized in vivo functions of MPYS and advanced our understanding of CDG as a mucosal vaccine adjuvant.
Keywords: STING; cell biology; dendritic cells; immunology; lung; mouse; mucosal adjuvant; pneumococcal vaccine.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

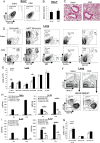

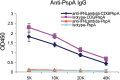






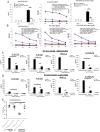

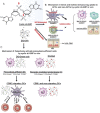

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

