Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin
- PMID: 25898123
- PMCID: PMC4412227
- DOI: 10.3201/eid2105.141626
Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin
Abstract
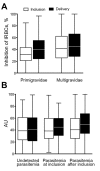
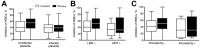
Placental malaria is caused by Plasmodium falciparum-infected erythrocytes that bind to placental tissue. Binding is mediated by VAR2CSA, a parasite antigen coded by the var gene, which interacts with chondroitin sulfate A (CSA). Consequences include maternal anemia and fetal growth retardation. Antibody-mediated immunity to placental malaria is acquired during successive pregnancies, but the target of VAR2CSA-specific protective antibodies is unclear. We assessed VAR2CSA-specific antibodies in pregnant women and analyzed their relationships with protection against placental infection, preterm birth, and low birthweight. Antibody responses to the N-terminal region of VAR2CSA during early pregnancy were associated with reduced risks for infections and low birthweight. Among women infected during pregnancy, an increase in CSA binding inhibition was associated with reduced risks for placental infection, preterm birth, and low birthweight. These data suggest that antibodies against VAR2CSA N-terminal region mediate immunity to placental malaria and associated outcomes. Our results validate current vaccine development efforts with VAR2CSA N-terminal constructs.
Keywords: Benin; Plasmodium falciparum; VAR2CSA; antibodies; erythrocytes; malaria; outcomes; parasites; placental malaria; pregnancy.
Figures


Similar articles
-
Plasmodium falciparum VAR2CSA-Specific IgG Subclass Responses Reflect Protection Against Low Birth Weight and Pregnancy-Associated Malaria.Front Immunol. 2021 Apr 21;12:610305. doi: 10.3389/fimmu.2021.610305. eCollection 2021. Front Immunol. 2021. PMID: 33968015 Free PMC article.
-
High levels of antibodies to multiple domains and strains of VAR2CSA correlate with the absence of placental malaria in Cameroonian women living in an area of high Plasmodium falciparum transmission.Infect Immun. 2012 Apr;80(4):1479-90. doi: 10.1128/IAI.00071-12. Epub 2012 Feb 13. Infect Immun. 2012. PMID: 22331427 Free PMC article.
-
The antibody response of pregnant Cameroonian women to VAR2CSA ID1-ID2a, a small recombinant protein containing the CSA-binding site.PLoS One. 2014 Feb 4;9(2):e88173. doi: 10.1371/journal.pone.0088173. eCollection 2014. PLoS One. 2014. PMID: 24505415 Free PMC article.
-
Designing a VAR2CSA-based vaccine to prevent placental malaria.Vaccine. 2015 Dec 22;33(52):7483-8. doi: 10.1016/j.vaccine.2015.10.011. Epub 2015 Nov 26. Vaccine. 2015. PMID: 26469717 Free PMC article. Review.
-
VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria.Front Immunol. 2021 Feb 9;11:624126. doi: 10.3389/fimmu.2020.624126. eCollection 2020. Front Immunol. 2021. PMID: 33633743 Free PMC article. Review.
Cited by
-
Cryo-EM reveals the conformational epitope of human monoclonal antibody PAM1.4 broadly reacting with polymorphic malarial protein VAR2CSA.PLoS Pathog. 2022 Nov 16;18(11):e1010924. doi: 10.1371/journal.ppat.1010924. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36383559 Free PMC article.
-
Mechanical forces control the valency of the malaria adhesin VAR2CSA by exposing cryptic glycan binding sites.PLoS Comput Biol. 2023 Dec 20;19(12):e1011726. doi: 10.1371/journal.pcbi.1011726. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38117828 Free PMC article.
-
Antibody levels to recombinant VAR2CSA domains vary with Plasmodium falciparum parasitaemia, gestational age, and gravidity, but do not predict pregnancy outcomes.Malar J. 2018 Mar 9;17(1):106. doi: 10.1186/s12936-018-2258-9. Malar J. 2018. PMID: 29523137 Free PMC article.
-
Association between malaria immunity and pregnancy outcomes among Malawian pregnant women receiving nutrient supplementation.Malar J. 2016 Nov 9;15(1):547. doi: 10.1186/s12936-016-1597-7. Malar J. 2016. PMID: 27829430 Free PMC article.
-
The Influence of Sub-Unit Composition and Expression System on the Functional Antibody Response in the Development of a VAR2CSA Based Plasmodium falciparum Placental Malaria Vaccine.PLoS One. 2015 Sep 1;10(9):e0135406. doi: 10.1371/journal.pone.0135406. eCollection 2015. PLoS One. 2015. PMID: 26327283 Free PMC article.
References
-
- Muthusamy A, Achur RN, Bhavanandan VP, Fouda GG, Taylor DW, Gowda DC. Plasmodium falciparum–infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am J Pathol. 2004;164:2013–25. 10.1016/S0002-9440(10)63761-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

