Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis
- PMID: 25898844
- PMCID: PMC4543785
- DOI: 10.1093/eurheartj/ehv109
Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis
Abstract
Aims: Osteopontin (OPN) is a multifunctional cytokine critically involved in cardiac fibrosis. However, the underlying mechanisms are unresolved. Non-coding RNAs are powerful regulators of gene expression and thus might mediate this process.
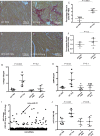
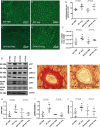
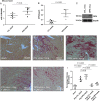
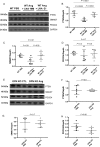
Methods and results: OPN and miR-21 were significantly increased in cardiac biopsies of patients with myocardial fibrosis. Ang II infusion via osmotic minipumps led to specific miRNA regulations with miR-21 being strongly induced in wild-type (WT) but not OPN knockout (KO) mice. This was associated with enhanced cardiac collagen content, myofibroblast activation, ERK-MAP kinase as well as AKT signalling pathway activation and a reduced expression of Phosphatase and Tensin Homologue (PTEN) as well as SMAD7 in WT but not OPN KO mice. In contrast, cardiotropic AAV9-mediated overexpression of OPN in vivo further enhanced cardiac fibrosis. In vitro, Ang II induced expression of miR-21 in WT cardiac fibroblasts, while miR-21 levels were unchanged in OPN KO fibroblasts. As pri-miR-21 was also increased by Ang II, we studied potential involved upstream regulators; Electrophoretic Mobility Shift and Chromatin Immunoprecipitation analyses confirmed activation of the miR-21 upstream-transcription factor AP-1 by Ang II. Recombinant OPN directly activated miR-21, enhanced fibrosis, and activated the phosphoinositide 3-kinase pathway. Locked nucleic acid-mediated miR-21 silencing ameliorated cardiac fibrosis development in vivo.
Conclusion: In cardiac fibrosis related to Ang II, miR-21 is transcriptionally activated and targets PTEN/SMAD7 resulting in increased fibroblast survival. OPN KO animals are protected from miR-21 increase and fibrosis development due to impaired AP-1 activation and fibroblast activation.
Keywords: Angiotensin II; Cardiac fibrosis; Osteopontin; miR-21; microRNA.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
miR-21 and cardiac fibrosis: another brick in the wall?Eur Heart J. 2015 Aug 21;36(32):2139-41. doi: 10.1093/eurheartj/ehv184. Epub 2015 May 13. Eur Heart J. 2015. PMID: 25975660 No abstract available.
References
-
- Thum T, Lorenzen JM. Cardiac fibrosis revisited by microRNA therapeutics. Circulation 2012;126:800–802. - PubMed
-
- Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin – a molecule for all seasons. QJM 2002;95:3–13. - PubMed
-
- Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res 2008;102:319–327. - PubMed
-
- Singh K, Sirokman G, Communal C, Robinson KG, Conrad CH, Brooks WW, Bing OH, Colucci WS. Myocardial osteopontin expression coincides with the development of heart failure. Hypertension 1999;33:663–670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

