Evaluation of cortical plasticity in children with cerebral palsy undergoing constraint-induced movement therapy based on functional near-infrared spectroscopy
- PMID: 25900145
- PMCID: PMC4479242
- DOI: 10.1117/1.JBO.20.4.046009
Evaluation of cortical plasticity in children with cerebral palsy undergoing constraint-induced movement therapy based on functional near-infrared spectroscopy
Abstract
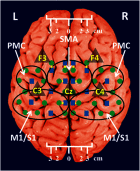
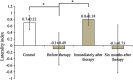
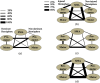
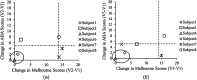
Sensorimotor cortex plasticity induced by constraint-induced movement therapy (CIMT) in six children (10.2±2.1 years old) with hemiplegic cerebral palsy was assessed by functional near-infrared spectroscopy (fNIRS). The activation laterality index and time-to-peak/duration during a finger-tapping task and the resting-state functional connectivity were quantified before, immediately after, and 6 months after CIMT. These fNIRS-based metrics were used to help explain changes in clinical scores of manual performance obtained concurrently with imaging time points. Five age-matched healthy children (9.8±1.3 years old) were also imaged to provide comparative activation metrics for normal controls. Interestingly, the activation time-to-peak/duration for all sensorimotor centers displayed significant normalization immediately after CIMT that persisted 6 months later. In contrast to this improved localized activation response, the laterality index and resting-state connectivity metrics that depended on communication between sensorimotor centers improved immediately after CIMT, but relapsed 6 months later. In addition, for the subjects measured in this work, there was either a trade-off between improving unimanual versus bimanual performance when sensorimotor activation patterns normalized after CIMT, or an improvement occurred in both unimanual and bimanual performance but at the cost of very abnormal plastic changes in sensorimotor activity.
Figures


References
-
- Rosenbaum P., et al. , “A report: the definition and classification of cerebral palsy April 2006,” Dev. Med. Child Neurol. Suppl. 109(suppl 109), 8–14 (2007). - PubMed
-
- Houwink A., et al. , “A neurocognitive perspective on developmental disregard in children with hemiplegic cerebral palsy,” Res. Dev. Disabil. 32(6), 2157–2163 (2011).RDDIEF - PubMed
-
- Van de Winckel A., et al. , “How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study,” Res. Dev. Disabil. 34(1), 183–197 (2013).RDDIEF - PubMed

