Characterization of plants expressing the human β1,4-galactosyltrasferase gene
- PMID: 25900423
- PMCID: PMC4451504
- DOI: 10.1016/j.plaphy.2015.04.010
Characterization of plants expressing the human β1,4-galactosyltrasferase gene
Abstract
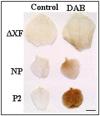
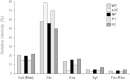
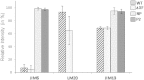
Modification of the plant N-glycosylation pathway towards human type structures is an important strategy to implement plants as expression systems for therapeutic proteins. Nevertheless, relatively little is known about the overall impact of non-plant glycosylation enzymes in stable transformed plants. Here, we analyzed transgenic lines (Nicotiana benthamiana and Arabidopsis thaliana) that stably express a modified version of human β1,4-galactosyltransferase ((ST)GalT). While some transgenic plants grew normally, other lines exhibited a severe phenotype associated with stunted growth and developmental retardation. The severity of the phenotype correlated with both increased (ST)GalT mRNA and protein levels but no differences were observed between N-glycosylation profiles of plants with and without the phenotype. In contrast to non-transgenic plants, all (ST)GalT expressing plants synthesized significant amounts of incompletely processed (largely depleted of core fucose) N-glycans with up to 40% terminally galactosylated structures. While transgenic plants showed no differences in nucleotide sugar composition and cell wall monosaccharide content, alterations in the reactivity of cell wall carbohydrate epitopes associated with arabinogalactan-proteins and pectic homogalacturonan were detected in (ST)GalT expressing plants. Notably, plants with phenotypic alterations showed increased levels of hydrogen peroxide, most probably a consequence of hypersensitive reactions. Our data demonstrate that unfavorable phenotypical modifications may occur upon stable in planta expression of non-native glycosyltransferases. Such important issues need to be taken into consideration in respect to stable glycan engineering in plants.
Keywords: Developmental phenotype; Glyco-engineering; Nicotiana benthamiana; Transgenic plants; β1,4-Galactosylation.
Copyright © 2015 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Figures



References
-
- Anumula K.R. Quantitative determination of monosaccharides in glycoproteins by high-performance liquid chromatography with highly sensitive fluorescence detection. Anal. Biochem. 1994;220:275–283. - PubMed
-
- Bakker H., Rouwendal G.J., Karnoup A.S., Florack D.E., Stoopen G.M., Helsper J.P., van Ree R., van Die I., Bosch D. An antibody produced in tobacco expressing a hybrid beta-1,4-galactosyltransferase is essentially devoid of plant carbohydrate epitopes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7577–7582. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

