Fabrication of 3-dimensional multicellular microvascular structures
- PMID: 25900808
- PMCID: PMC4511194
- DOI: 10.1096/fj.14-263343
Fabrication of 3-dimensional multicellular microvascular structures
Abstract
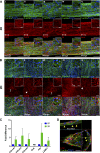
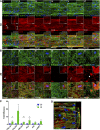
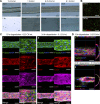
Despite current advances in engineering blood vessels over 1 mm in diameter and the existing wealth of knowledge regarding capillary bed formation, studies for the development of microvasculature, the connecting bridge between them, have been extremely limited so far. Here, we evaluate the use of 3-dimensional (3D) microfibers fabricated by hydrogel electrospinning as templates for microvascular structure formation. We hypothesize that 3D microfibers improve extracellular matrix (ECM) deposition from vascular cells, enabling the formation of freestanding luminal multicellular microvasculature. Compared to 2-dimensional cultures, we demonstrate with confocal microscopy and RT-PCR that fibrin microfibers induce an increased ECM protein deposition by vascular cells, specifically endothelial colony-forming cells, pericytes, and vascular smooth muscle cells. These ECM proteins comprise different layers of the vascular wall including collagen types I, III, and IV, as well as elastin, fibronectin, and laminin. We further demonstrate the achievement of multicellular microvascular structures with an organized endothelium and a robust multicellular perivascular tunica media. This, along with the increased ECM deposition, allowed for the creation of self-supporting multilayered microvasculature with a distinct circular lumen following fibrin microfiber core removal. This approach presents an advancement toward the development of human microvasculature for basic and translational studies.
Keywords: endothelial cell; extracellular matrix; fibrin; microfiber; perivascular cell.
© FASEB.
Figures



References
-
- Huang G. Y., Zhou L. H., Zhang Q. C., Chen Y. M., Sun W., Xu F., Lu T. J. (2011) Microfluidic hydrogels for tissue engineering. Biofabrication 3, 012001 - PubMed
-
- Auger F. A., Gibot L., Lacroix D. (2013) The pivotal role of vascularization in tissue engineering. Annu. Rev. Biomed. Eng. 15, 177–200 - PubMed
-
- Roy S., Ha J., Trudeau K., Beglova E. (2010) Vascular basement membrane thickening in diabetic retinopathy. Curr. Eye Res. 35, 1045–1056 - PubMed
-
- Hibbs R. G., Burch G. E., Phillips J. H. (1958) The fine structure of the small blood vessels of normal human dermis and subcutis. Am. Heart J. 56, 662–670 - PubMed
-
- Yen A., Braverman I. M. (1976) Ultrastructure of the human dermal microcirculation: the horizontal plexus of the papillary dermis. J. Invest. Dermatol. 66, 131–142 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

