Carotid body overactivity induces respiratory neurone channelopathy contributing to neurogenic hypertension
- PMID: 25900825
- PMCID: PMC4532526
- DOI: 10.1113/JP270423
Carotid body overactivity induces respiratory neurone channelopathy contributing to neurogenic hypertension
Abstract
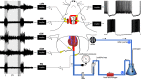
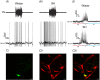
Why sympathetic activity rises in neurogenic hypertension remains unknown. It has been postulated that changes in the electrical excitability of medullary pre-sympathetic neurones are the main causal mechanism for the development of sympathetic overactivity in experimental hypertension. Here we review recent data suggesting that enhanced sympathetic activity in neurogenic hypertension is, at least in part, dependent on alterations in the electrical excitability of medullary respiratory neurones and their central modulation of sympatho-excitatory networks. We also present results showing a critical role for carotid body tonicity in the aetiology of enhanced central respiratory modulation of sympathetic activity in neurogenic hypertension. We propose a novel hypothesis of respiratory neurone channelopathy induced by carotid body overactivity in neurogenic hypertension that may contribute to sympathetic excess. Moreover, our data support the notion of targeting the carotid body as a potential novel therapeutic approach for reducing sympathetic vasomotor tone in neurogenic hypertension.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures

References
-
- Abdala AP, Paton JF. Smith JC. Defining inhibitory neurone function in respiratory circuits: opportunities with optogenetics? J Physiol. 2014 (in press; DOI: 10.1113/jphysiol.2014.280610 ) - DOI - PMC - PubMed
-
- Almado CE, Leao RM. Machado BH. Intrinsic properties of rostral ventrolateral medulla presympathetic and bulbospinal respiratory neurons of juvenile rats are not affected by chronic intermittent hypoxia. Exp Physiol. 2014;99:937–950. - PubMed
-
- Bergamaschi C, Campos RR, Schor N. Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–1120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

