Regulation of thrombosis and vascular function by protein methionine oxidation
- PMID: 25900980
- PMCID: PMC4473114
- DOI: 10.1182/blood-2015-01-544676
Regulation of thrombosis and vascular function by protein methionine oxidation
Abstract
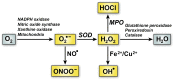
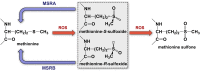
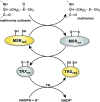
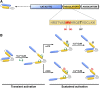
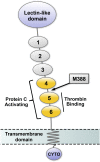
Redox biology is fundamental to both normal cellular homeostasis and pathological states associated with excessive oxidative stress. Reactive oxygen species function not only as signaling molecules but also as redox regulators of protein function. In the vascular system, redox reactions help regulate key physiologic responses such as cell adhesion, vasoconstriction, platelet aggregation, angiogenesis, inflammatory gene expression, and apoptosis. During pathologic states, altered redox balance can cause vascular cell dysfunction and affect the equilibrium between procoagulant and anticoagulant systems, contributing to thrombotic vascular disease. This review focuses on the emerging role of a specific reversible redox reaction, protein methionine oxidation, in vascular disease and thrombosis. A growing number of cardiovascular and hemostatic proteins are recognized to undergo reversible methionine oxidation, in which methionine residues are posttranslationally oxidized to methionine sulfoxide. Protein methionine oxidation can be reversed by the action of stereospecific enzymes known as methionine sulfoxide reductases. Calcium/calmodulin-dependent protein kinase II is a prototypical methionine redox sensor that responds to changes in the intracellular redox state via reversible oxidation of tandem methionine residues in its regulatory domain. Several other proteins with oxidation-sensitive methionine residues, including apolipoprotein A-I, thrombomodulin, and von Willebrand factor, may contribute to vascular disease and thrombosis.
© 2015 by The American Society of Hematology.
Figures
References
-
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

