Safe and Effective Use of the Once Weekly Dulaglutide Single-Dose Pen in Injection-Naïve Patients With Type 2 Diabetes
- PMID: 25901022
- PMCID: PMC4667342
- DOI: 10.1177/1932296815583059
Safe and Effective Use of the Once Weekly Dulaglutide Single-Dose Pen in Injection-Naïve Patients With Type 2 Diabetes
Abstract
Background: This 4-week, phase 3b, multicenter, open-label, single-arm, outpatient study demonstrated the safe and effective use of the dulaglutide single-dose pen containing 0.5 mL of placebo for subcutaneous injection in injection-naïve adult patients with type 2 diabetes (T2D), with A1C ≤ 8.5% (69 mmol/mol), BMI ≥ 23 kg/m2 and ≤ 45 kg/m(2).
Method: Patients completed a modified self-injecting subscale of the Diabetes Fear of Injecting and Self-Testing Questionnaire (mD-FISQ) and were trained to self-inject with the single-dose pen. Patients completed the initial self-injection at the site, injected at home for 2 subsequent weeks, and returned to the site for the final injection. The initial and final self-injections were evaluated for success; the final (initial) self-injection success rate was the primary (secondary) outcome measure, and the primary (secondary) objective was to demonstrate this success rate as being significantly greater than 80%. Patients recorded their level of pain after each injection. After the final injection, patients completed the mD-FISQ and the Medication Delivery Device Assessment Battery (MDDAB) to assess their perceptions of the single-dose pen, including ease of use and experience with the device.
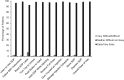
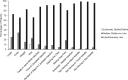
Results: Among 211 patients (mean age: 61 years), the primary objective was met, with a final injection success rate of 99.1% (95% CI: 96.6% to 99.7%). Among 214 patients, the initial injection success rate was 97.2% (95% CI: 94.0% to 98.7%), meeting the key secondary objective. Overall, most patients (>96%) found the device easy to use, were satisfied with the device, and would be willing to continue to use the single-dose pen after the study. There was a significant reduction (P < .001) from baseline to study end in patients' fear of self-injecting, as measured by the mD-FISQ.
Conclusions: The dulaglutide single-dose pen was found to be a safe and effective device for use by patients with T2D who were injection-naïve. A positive injection experience is an important factor for patients and providers when initiating injectable therapy.
Keywords: dulaglutide; self-injection; single-dose pen; type 2 diabetes.
© 2015 Diabetes Technology Society.
Conflict of interest statement
Figures



References
-
- Peyrot M, Matthews DR, Snoek FJ, et al. An international study of psychological resistance to insulin use among persons with diabetes. Diabetologia. 2003;46(suppl 2):A89.
-
- Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679. - PubMed
-
- Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(suppl 3):S18-S24. - PubMed
-
- An J, Nichol MB. Multiple medication adherence and its effect on clinical outcomes among patients with comorbid type 2 diabetes and hypertension. Med Care. 2013;51:879-887. - PubMed
-
- Hornsten A, Lundman B, Selstam EK, Sandstrom H. Patient satisfaction with diabetes care. J Adv Nurs. 2005;51:609-617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

