AZFc deletions do not affect the function of human spermatogonia in vitro
- PMID: 25901025
- PMCID: PMC5009458
- DOI: 10.1093/molehr/gav022
AZFc deletions do not affect the function of human spermatogonia in vitro
Abstract
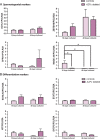
Azoospermic factor c (AZFc) deletions are the underlying cause in 10% of azoo- or severe oligozoospermia. Through extensive molecular analysis the precise genetic content of the AZFc region and the origin of its deletion have been determined. However, little is known about the effect of AZFc deletions on the functionality of germ cells at various developmental steps. The presence of normal, fertilization-competent sperm in the ejaculate and/or testis of the majority of men with AZFc deletions suggests that the process of differentiation from spermatogonial stem cells (SSCs) to mature spermatozoa can take place in the absence of the AZFc region. To determine the functionality of AZFc-deleted spermatogonia, we compared in vitro propagated spermatogonia from six men with complete AZFc deletions with spermatogonia from three normozoospermic controls. We found that spermatogonia of AZFc-deleted men behave similar to controls during culture. Short-term (18 days) and long-term (48 days) culture of AZFc-deleted spermatogonia showed the same characteristics as non-deleted spermatogonia. This similarity was revealed by the same number of passages, the same germ cell clusters formation and similar level of genes expression of spermatogonial markers including ubiquitin carboxyl-terminal esterase L1 (UCHL1), zinc finger and BTB domain containing 16 (ZBTB16) and glial cell line-derived neurotrophic factor family receptor alpha 1 (GFRA1), as well as germ cell differentiation markers including signal transducer and activator of transcription 3 (STAT3), spermatogenesis and oogenesis specific basic helix-loophelix 2 (SOHLH2), v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) and synaptonemal complex protein 3 (SYCP3). The only exception was melanoma antigen family A4 (MAGEA4) which showed significantly lower expression in AZFc-deleted samples than controls in short-term culture while in long-term culture it was hardly detected in both AZFc-deleted and control spermatogonia. These data suggest that, at least in vitro, spermatogonia of AZFc-deleted men are functionally similar to spermatogonia from non-deleted men. Potentially, this enables treatment of men with AZFc deletions by propagating their SSCs in vitro and autotransplanting these SSCs back to the testes to increase sperm counts and restore fertility.
Keywords: AZFc; SSC; infertility; spermatogonia.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems.Acta Histochem. 2020 Dec;122(8):151627. doi: 10.1016/j.acthis.2020.151627. Epub 2020 Sep 28. Acta Histochem. 2020. PMID: 33002788
-
The testicular transcriptome associated with spermatogonia differentiation initiated by gonadotrophin stimulation in the juvenile rhesus monkey (Macaca mulatta).Hum Reprod. 2017 Oct 1;32(10):2088-2100. doi: 10.1093/humrep/dex270. Hum Reprod. 2017. PMID: 28938749 Free PMC article.
-
Characterization and in vitro culture of putative spermatogonial stem cells derived from feline testicular tissue.J Reprod Dev. 2013;59(2):189-95. doi: 10.1262/jrd.2012-130. Epub 2013 Jan 25. J Reprod Dev. 2013. PMID: 23358308 Free PMC article.
-
Polymorphic DAZ gene family in polymorphic structure of AZFc locus: Artwork or functional for human spermatogenesis?APMIS. 2003 Jan;111(1):115-26; discussion 126-7. doi: 10.1034/j.1600-0463.2003.11101161.x. APMIS. 2003. PMID: 12752250 Review.
-
Age-related presence of spermatogonia in patients with Klinefelter syndrome: a systematic review and meta-analysis.Hum Reprod Update. 2020 Jan 1;26(1):58-72. doi: 10.1093/humupd/dmz038. Hum Reprod Update. 2020. PMID: 31822886
Cited by
-
Natural Transmission of b2/b3 Subdeletion or Duplication to Expanded Y Chromosome Microdeletions.Med Sci Monit. 2018 Sep 18;24:6559-6563. doi: 10.12659/MSM.911644. Med Sci Monit. 2018. PMID: 30226219 Free PMC article.
-
Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases.Hum Reprod Update. 2016 Sep;22(5):561-73. doi: 10.1093/humupd/dmw017. Epub 2016 May 30. Hum Reprod Update. 2016. PMID: 27240817 Free PMC article. Review.
-
DAZL mediates a broad translational program regulating expansion and differentiation of spermatogonial progenitors.Elife. 2020 Jul 20;9:e56523. doi: 10.7554/eLife.56523. Elife. 2020. PMID: 32686646 Free PMC article.
-
Infertility treatment for patients having a microdeletion of azoospermic factor (AZF).Nagoya J Med Sci. 2023 May;85(2):233-240. doi: 10.18999/nagjms.85.2.233. Nagoya J Med Sci. 2023. PMID: 37346843 Free PMC article.
-
In Vitro Modeling of Human Germ Cell Development Using Pluripotent Stem Cells.Stem Cell Reports. 2018 Feb 13;10(2):509-523. doi: 10.1016/j.stemcr.2018.01.001. Epub 2018 Feb 1. Stem Cell Reports. 2018. PMID: 29398481 Free PMC article.
References
-
- Aarabi M, Modarressi MH, Soltanghoraee H, Behjati R, Amirjannati N, Akhondi MM. Testicular expression of synaptonemal complex protein 3 (SYCP3) messenger ribonucleic acid in 110 patients with nonobstructive azoospermia. Fertil Steril 2006;86:325–331. - PubMed
-
- Anderson DJ, Narayan P, DeWolf WC. Major histocompatibility antigens are not detectable on post-meiotic human testicular germ cells. J Immunol 1984;133:1962–1965. - PubMed
-
- Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod 2005;73:1011–1016. - PubMed
-
- Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril 2009;91:2281–2294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

