60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis
- PMID: 25901041
- PMCID: PMC4498991
- DOI: 10.1530/JOE-15-0113
60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis
Abstract
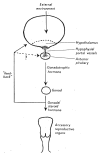
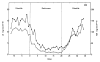
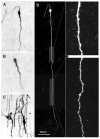
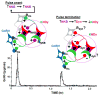
This review provides an outline of how our understanding of the neuroendocrine control of the hypothalamo-pituitary-gonadal axis has evolved since the publication of Geoffrey Harris' renowned monograph in 1955. Particular attention is directed to the neurobiology underlying pulsatile GnRH release from the hypothalamus, the neuroendocrine control of ovarian cycles, puberty and seasonality of gonadal function, and to ideas that have emerged as a result of examining the relationship between growth and the reproductive axis. The review closes with i) a brief discussion of how knowledge gained as a result of pursuing the early hypotheses of Harris has led to major clinical and therapeutic applications, and ii) a personal glimpse into the future of research in this fascinating area of biology.
Keywords: gonadotrophin releasing hormone; hypothalamus; neuroendocrinology; ovulation; reproduction.
© 2015 Society for Endocrinology.
Conflict of interest statement
Figures

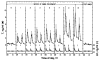
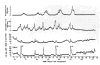

References
-
- Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44:205–210. - PubMed
-
- Barry J, Dubois MP, Poulain P. LRF producing cells of the mammalian hypothalamus. A fluorescent antibody study. Z Zellforsch Mikrosk Anat. 1973;146:351–366. - PubMed
-
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. - PubMed
-
- Clark SJ, Ellis N, Styne DM, Gluckman PD, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. XVII. Demonstration of pulsatile luteinizing hormone secretion by the fetal pituitary gland. Endocrinology. 1984;115:1774–1779. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

