Endoscopic ultrasound in the evaluation of pancreatic neoplasms-solid and cystic: A review
- PMID: 25901210
- PMCID: PMC4400620
- DOI: 10.4253/wjge.v7.i4.318
Endoscopic ultrasound in the evaluation of pancreatic neoplasms-solid and cystic: A review
Abstract
Pancreatic neoplasms have a wide range of pathology, from pancreatic adenocarcinoma to cystic mucinous neoplasms. Endoscopic ultrasound (EUS) with or without fine needle aspiration (FNA) is a helpful diagnostic tool in the work-up of pancreatic neoplasms. Its utility in pancreatic malignancy is well known. Over the last two decades EUS-FNA has become a procedure of choice for diagnosis of pancreatic adenocarcinoma. EUS-FNA is highly sensitive and specific for solid lesions, with sensitivities as high as 80%-95% for pancreatic masses and specificity as high as 75%-100%. Multiple aspects of the procedure have been studied to optimize the rate of diagnosis with EUS-FNA including cytopathologist involvement, needle size, suctioning and experience of endoscopist. Onsite pathology is one of the most important elements in increasing diagnostic yield rate in EUS-FNA. EUS-FNA is valuable in diagnosing rare and atypical pancreatic neoplasms including neuroendocrine, lymphoma and metastatic disease. As more and more patients undergo cross sectional imaging, cystic lesions of the pancreas are becoming a more common occurrence and EUS-FNA of these lesions can be helpful for differentiation. This review covers the technical aspects of optimizing pancreatic neoplasm diagnosis rate, highlight rare pancreatic neoplasms and role of EUS-FNA, and also outline the important factors in diagnosis of cystic lesions by EUS-FNA.
Keywords: Endoscopic ultrasound-fine needle aspiration; Pancreatic adenocarcinoma; Pancreatic cysts; Pancreatic neoplasms; Review.
Figures

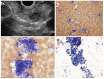
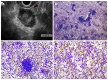




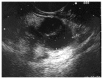
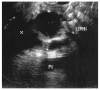

References
-
- Helmstaedter L, Riemann JF. Pancreatic cancer--EUS and early diagnosis. Langenbecks Arch Surg. 2008;393:923–927. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–861. - PubMed
-
- Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717–725; quiz 664. - PubMed
-
- Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–173. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

