Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results
- PMID: 25901286
- PMCID: PMC4404644
- DOI: 10.1186/s40425-015-0055-3
Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results
Abstract
Background: AGS-003 is an autologous immunotherapy prepared from fully matured and optimized monocyte-derived dendritic cells, which are co-electroporated with amplified tumor RNA plus synthetic CD40L RNA. AGS-003 was evaluated in combination with sunitinib in an open label phase 2 study in intermediate and poor risk, treatment naïve patients with metastatic clear cell renal cell carcinoma (mRCC).
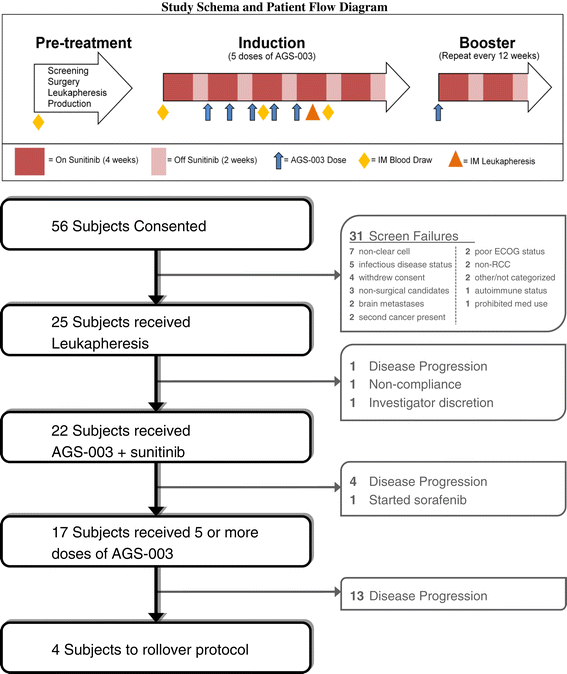
Methods: Twenty-one intermediate and poor risk patients were treated continuously with sunitinib (4 weeks on, 2 weeks off per 6 week cycle). After completion of the first cycle of sunitinib, patients were treated with AGS-003 every 3 weeks for 5 doses, then every 12 weeks until progression or end of study. The primary endpoint was to determine the complete response rate. Secondary endpoints included clinical benefit, safety, progression free survival (PFS) and overall survival (OS). Immunologic response was also monitored.
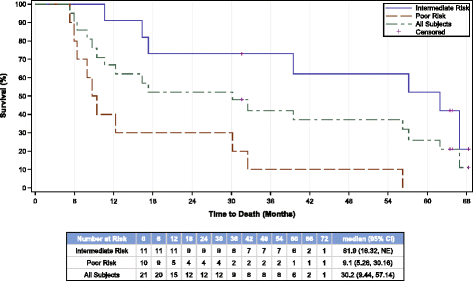
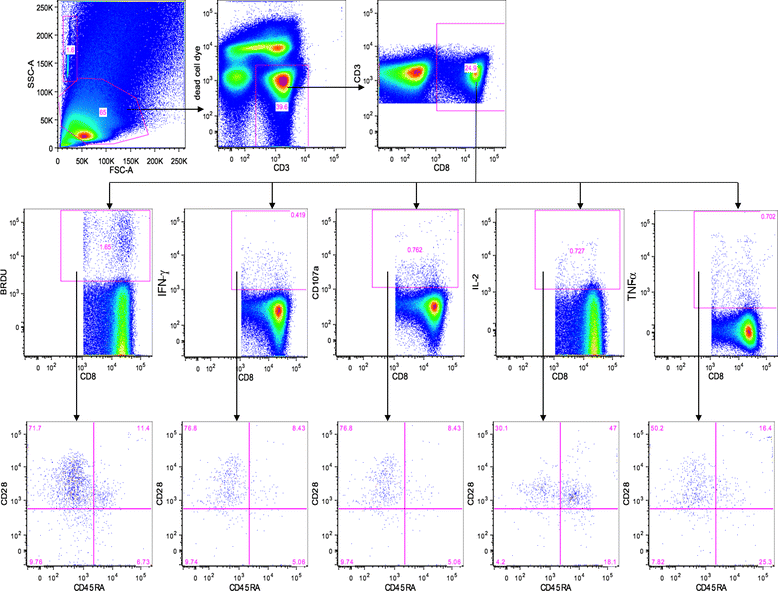
Results: Thirteen patients (62%) experienced clinical benefit (9 partial responses, 4 with stable disease); however there were no complete responses in this group of intermediate and poor risk mRCC patients and enrollment was terminated early. Median PFS from registration was 11.2 months (95% CI 6.0, 19.4) and the median OS from registration was 30.2 months (95% CI 9.4, 57.1) for all patients. Seven (33%) patients survived for at least 4.5 years, while five (24%) survived for more than 5 years, including 2 patients who remain progression-free with durable responses for more than 5 years at the time of this report. AGS-003 was well tolerated with only mild injection-site reactions. The most common adverse events were related to expected toxicity from sunitinib therapy. In patients who had sequential samples available for immune monitoring, the magnitude of the increase in the absolute number of CD8(+) CD28(+) CD45RA(-) effector/memory T cells (CTLs) after 5 doses of AGS-003 relative to baseline, correlated with overall survival.
Conclusions: AGS-003 in combination with sunitinib was well tolerated and yielded supportive immunologic responses coupled with extension of median and long-term survival in an unselected, intermediate and poor risk prognosis mRCC population.
Clinical trial registry: #NCT00678119.
Keywords: AGS-003; Dendritic cell; Immunotherapy; RCC; Sunitinib.
Figures
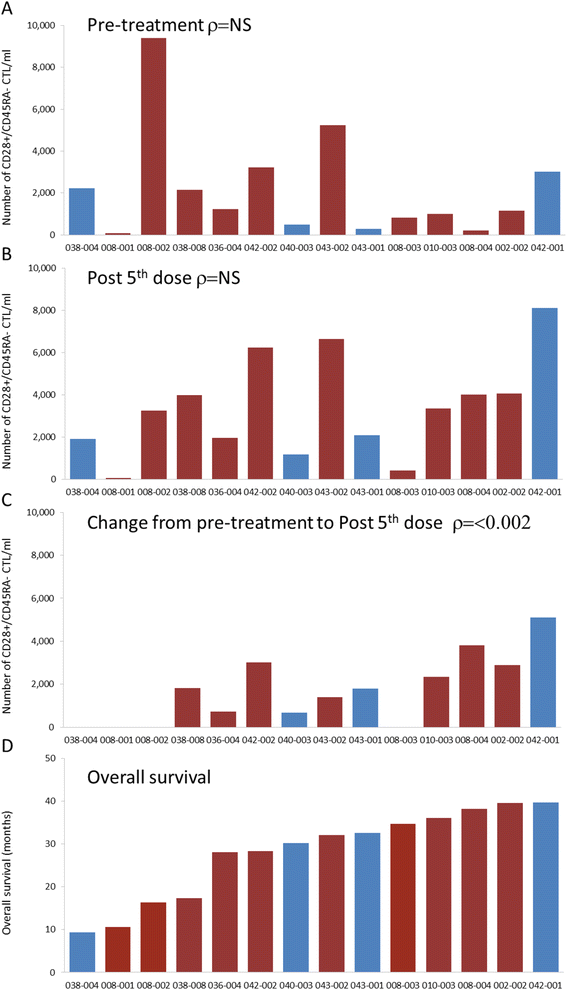
 ) identify intermediate risk patients and red bars (
) identify intermediate risk patients and red bars ( ) identify poor risk patients. The correlation between the absolute number of CD28+/CD45RA- CTLs and overall survival was statistically significant by nonparametric bivariate analysis, Spearman’s ρ = 0.8; p < 0.002.
) identify poor risk patients. The correlation between the absolute number of CD28+/CD45RA- CTLs and overall survival was statistically significant by nonparametric bivariate analysis, Spearman’s ρ = 0.8; p < 0.002.References
-
- Albiges L, Oudard S, Negrier S, Caty A, Gravis G, Joly F, Duclos B, Geoffrois L, Rolland F, Guillot A, Laguerre B, Legouffe E, Kohser F, Dietrich PY, Theodore CA, Escudier B. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482–487. doi: 10.1200/JCO.2011.37.2516. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
