Implausibility of the vibrational theory of olfaction
- PMID: 25901328
- PMCID: PMC4450420
- DOI: 10.1073/pnas.1503054112
Implausibility of the vibrational theory of olfaction
Abstract
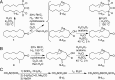
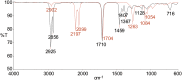
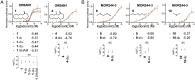
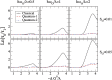
The vibrational theory of olfaction assumes that electron transfer occurs across odorants at the active sites of odorant receptors (ORs), serving as a sensitive measure of odorant vibrational frequencies, ultimately leading to olfactory perception. A previous study reported that human subjects differentiated hydrogen/deuterium isotopomers (isomers with isotopic atoms) of the musk compound cyclopentadecanone as evidence supporting the theory. Here, we find no evidence for such differentiation at the molecular level. In fact, we find that the human musk-recognizing receptor, OR5AN1, identified using a heterologous OR expression system and robustly responding to cyclopentadecanone and muscone, fails to distinguish isotopomers of these compounds in vitro. Furthermore, the mouse (methylthio)methanethiol-recognizing receptor, MOR244-3, as well as other selected human and mouse ORs, responded similarly to normal, deuterated, and (13)C isotopomers of their respective ligands, paralleling our results with the musk receptor OR5AN1. These findings suggest that the proposed vibration theory does not apply to the human musk receptor OR5AN1, mouse thiol receptor MOR244-3, or other ORs examined. Also, contrary to the vibration theory predictions, muscone-d30 lacks the 1,380- to 1,550-cm(-1) IR bands claimed to be essential for musk odor. Furthermore, our theoretical analysis shows that the proposed electron transfer mechanism of the vibrational frequencies of odorants could be easily suppressed by quantum effects of nonodorant molecular vibrational modes. These and other concerns about electron transfer at ORs, together with our extensive experimental data, argue against the plausibility of the vibration theory.
Keywords: cyclopentadecanone; electron transfer; isotopomers; muscone; olfaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Laying a controversial smell theory to rest.Proc Natl Acad Sci U S A. 2015 May 26;112(21):6525-6. doi: 10.1073/pnas.1507103112. Epub 2015 May 18. Proc Natl Acad Sci U S A. 2015. PMID: 26015552 Free PMC article. No abstract available.
-
Reply to Turin et al.: Vibrational theory of olfaction is implausible.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):E3155. doi: 10.1073/pnas.1508443112. Epub 2015 Jun 4. Proc Natl Acad Sci U S A. 2015. PMID: 26045493 Free PMC article. No abstract available.
-
Plausibility of the vibrational theory of olfaction.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):E3154. doi: 10.1073/pnas.1508035112. Epub 2015 Jun 4. Proc Natl Acad Sci U S A. 2015. PMID: 26045494 Free PMC article. No abstract available.
References
-
- Sell CS. Chemistry and the Sense of Smell. Wiley; Hoboken, NJ: 2014.
-
- Moncrieff RW. The Chemical Senses. 3rd Ed Leonard Hill; London: 1967.
-
- Dyson GM. The scientific basis of odor. Chem Ind. 1938;57(28):647–651.
-
- Dyson GM. Some aspects of the vibration theory of odor. Perfumery and Essential Oil Record. 1928;19:456–459.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

