Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel
- PMID: 25901329
- PMCID: PMC4426425
- DOI: 10.1073/pnas.1503488112
Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel
Abstract
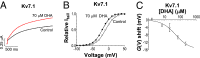
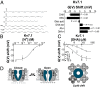
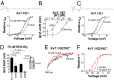
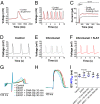
Polyunsaturated fatty acids (PUFAs) affect cardiac excitability. Kv7.1 and the β-subunit KCNE1 form the cardiac IKs channel that is central for cardiac repolarization. In this study, we explore the prospects of PUFAs as IKs channel modulators. We report that PUFAs open Kv7.1 via an electrostatic mechanism. Both the polyunsaturated acyl tail and the negatively charged carboxyl head group are required for PUFAs to open Kv7.1. We further show that KCNE1 coexpression abolishes the PUFA effect on Kv7.1 by promoting PUFA protonation. PUFA analogs with a decreased pKa value, to preserve their negative charge at neutral pH, restore the sensitivity to open IKs channels. PUFA analogs with a positively charged head group inhibit IKs channels. These different PUFA analogs could be developed into drugs to treat cardiac arrhythmias. In support of this possibility, we show that PUFA analogs act antiarrhythmically in embryonic rat cardiomyocytes and in isolated perfused hearts from guinea pig.
Keywords: IKs; KCNE1; KCNQ1; Kv7.1; antiarrhythmic.
Conflict of interest statement
Conflict of interest statement: A patent application based on these results has been submitted by the University of Miami with S.I.L., H.P.L., and F.E. identified as inventors.
Figures

Similar articles
-
KCNE1 tunes the sensitivity of KV7.1 to polyunsaturated fatty acids by moving turret residues close to the binding site.Elife. 2018 Jul 17;7:e37257. doi: 10.7554/eLife.37257. Elife. 2018. PMID: 30014849 Free PMC article.
-
PUFA stabilizes a conductive state of the selectivity filter in IKs channels.Elife. 2024 Oct 31;13:RP95852. doi: 10.7554/eLife.95852. Elife. 2024. PMID: 39480699 Free PMC article.
-
Polyunsaturated fatty acids produce a range of activators for heterogeneous IKs channel dysfunction.J Gen Physiol. 2020 Feb 3;152(2):e201912396. doi: 10.1085/jgp.201912396. J Gen Physiol. 2020. PMID: 31865382 Free PMC article.
-
Polyunsaturated Fatty Acids as Modulators of KV7 Channels.Front Physiol. 2020 Jun 11;11:641. doi: 10.3389/fphys.2020.00641. eCollection 2020. Front Physiol. 2020. PMID: 32595524 Free PMC article. Review.
-
Regulation of KCNQ/Kv7 family voltage-gated K+ channels by lipids.Biochim Biophys Acta Biomembr. 2017 Apr;1859(4):586-597. doi: 10.1016/j.bbamem.2016.10.023. Epub 2016 Nov 4. Biochim Biophys Acta Biomembr. 2017. PMID: 27818172 Free PMC article. Review.
Cited by
-
Regulation of membrane KCNQ1/KCNE1 channel density by sphingomyelin synthase 1.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C15-23. doi: 10.1152/ajpcell.00272.2015. Epub 2016 May 18. Am J Physiol Cell Physiol. 2016. PMID: 27194473 Free PMC article.
-
Suppression of ventricular arrhythmias by targeting late L-type Ca2+ current.J Gen Physiol. 2021 Dec 6;153(12):e202012584. doi: 10.1085/jgp.202012584. Epub 2021 Oct 26. J Gen Physiol. 2021. PMID: 34698805 Free PMC article.
-
SUMOylation determines the voltage required to activate cardiac IKs channels.Proc Natl Acad Sci U S A. 2017 Aug 8;114(32):E6686-E6694. doi: 10.1073/pnas.1706267114. Epub 2017 Jul 25. Proc Natl Acad Sci U S A. 2017. PMID: 28743749 Free PMC article.
-
Functions of the KCNE Gene Family in Ion Channels.Biochem Genet. 2025 Jul 16. doi: 10.1007/s10528-025-11202-3. Online ahead of print. Biochem Genet. 2025. PMID: 40668462 Review.
-
Reciprocal voltage sensor-to-pore coupling leads to potassium channel C-type inactivation.Sci Rep. 2016 Jun 9;6:27562. doi: 10.1038/srep27562. Sci Rep. 2016. PMID: 27278891 Free PMC article.
References
-
- Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85(4):1205–1253. - PubMed
-
- Börjesson SI, Elinder F. Structure, function, and modification of the voltage sensor in voltage-gated ion channels. Cell Biochem Biophys. 2008;52(3):149–174. - PubMed
-
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19(1):175–184. - PubMed
-
- Jiang Y, et al. The open pore conformation of potassium channels. Nature. 2002;417(6888):523–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

