Mutant MHC class II epitopes drive therapeutic immune responses to cancer
- PMID: 25901682
- PMCID: PMC4838069
- DOI: 10.1038/nature14426
Mutant MHC class II epitopes drive therapeutic immune responses to cancer
Erratum in
-
Erratum: Mutant MHC class II epitopes drive therapeutic immune responses to cancer.Nature. 2015 Jul 16;523(7560):370. doi: 10.1038/nature14567. Epub 2015 Jun 3. Nature. 2015. PMID: 26040715 No abstract available.
Abstract
Tumour-specific mutations are ideal targets for cancer immunotherapy as they lack expression in healthy tissues and can potentially be recognized as neo-antigens by the mature T-cell repertoire. Their systematic targeting by vaccine approaches, however, has been hampered by the fact that every patient's tumour possesses a unique set of mutations ('the mutanome') that must first be identified. Recently, we proposed a personalized immunotherapy approach to target the full spectrum of a patient's individual tumour-specific mutations. Here we show in three independent murine tumour models that a considerable fraction of non-synonymous cancer mutations is immunogenic and that, unexpectedly, the majority of the immunogenic mutanome is recognized by CD4(+) T cells. Vaccination with such CD4(+) immunogenic mutations confers strong antitumour activity. Encouraged by these findings, we established a process by which mutations identified by exome sequencing could be selected as vaccine targets solely through bioinformatic prioritization on the basis of their expression levels and major histocompatibility complex (MHC) class II-binding capacity for rapid production as synthetic poly-neo-epitope messenger RNA vaccines. We show that vaccination with such polytope mRNA vaccines induces potent tumour control and complete rejection of established aggressively growing tumours in mice. Moreover, we demonstrate that CD4(+) T cell neo-epitope vaccination reshapes the tumour microenvironment and induces cytotoxic T lymphocyte responses against an independent immunodominant antigen in mice, indicating orchestration of antigen spread. Finally, we demonstrate an abundance of mutations predicted to bind to MHC class II in human cancers as well by employing the same predictive algorithm on corresponding human cancer types. Thus, the tailored immunotherapy approach introduced here may be regarded as a universally applicable blueprint for comprehensive exploitation of the substantial neo-epitope target repertoire of cancers, enabling the effective targeting of every patient's tumour with vaccines produced 'just in time'.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper. Readers are welcome to comment on the online version of the paper.
Figures
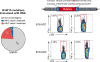
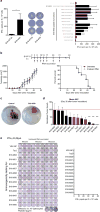
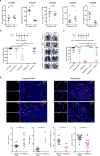
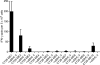



Comment in
-
Cancer immunotherapy: exploiting neoepitopes.Cell Res. 2015 Aug;25(8):887-8. doi: 10.1038/cr.2015.66. Epub 2015 Jun 2. Cell Res. 2015. PMID: 26032267 Free PMC article.
References
-
- Castle JC, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. - PubMed
-
- Holtkamp S, et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. - PubMed
-
- Kreiter S, et al. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J Immunol. 2008;180:309–318. - PubMed
-
- Kuhn AN, et al. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 2010;17:961–971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

