Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates
- PMID: 25901685
- PMCID: PMC4467030
- DOI: 10.1038/nature14442
Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates
Abstract
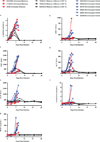
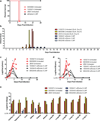
The current outbreak of Ebola virus in West Africa is unprecedented, causing more cases and fatalities than all previous outbreaks combined, and has yet to be controlled. Several post-exposure interventions have been employed under compassionate use to treat patients repatriated to Europe and the United States. However, the in vivo efficacy of these interventions against the new outbreak strain of Ebola virus is unknown. Here we show that lipid-nanoparticle-encapsulated short interfering RNAs (siRNAs) rapidly adapted to target the Makona outbreak strain of Ebola virus are able to protect 100% of rhesus monkeys against lethal challenge when treatment was initiated at 3 days after exposure while animals were viraemic and clinically ill. Although all infected animals showed evidence of advanced disease including abnormal haematology, blood chemistry and coagulopathy, siRNA-treated animals had milder clinical features and fully recovered, while the untreated control animals succumbed to the disease. These results represent the first, to our knowledge, successful demonstration of therapeutic anti-Ebola virus efficacy against the new outbreak strain in nonhuman primates and highlight the rapid development of lipid-nanoparticle-delivered siRNA as a countermeasure against this highly lethal human disease.
Figures
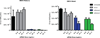



Comment in
-
Viral infections: New options to fight Ebola.Nat Rev Drug Discov. 2015 Jun;14(6):385. doi: 10.1038/nrd4642. Nat Rev Drug Discov. 2015. PMID: 26027535 No abstract available.
References
-
- World Health Organization. Ebola Data and Statistics. 2015 < http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-20150122?la...>.
-
- Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother. 2015;49:196–206. - PubMed
-
- Qiu X, et al. mAbs and Ad-vectored IFN-alpha therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med. 2013;5:207ra143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

