ASK1 restores the antiviral activity of APOBEC3G by disrupting HIV-1 Vif-mediated counteraction
- PMID: 25901786
- PMCID: PMC4423214
- DOI: 10.1038/ncomms7945
ASK1 restores the antiviral activity of APOBEC3G by disrupting HIV-1 Vif-mediated counteraction
Abstract
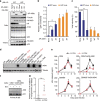
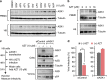
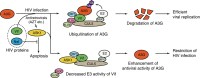
APOBEC3G (A3G) is an innate antiviral restriction factor that strongly inhibits the replication of human immunodeficiency virus type 1 (HIV-1). An HIV-1 accessory protein, Vif, hijacks the host ubiquitin-proteasome system to execute A3G degradation. Identification of the host pathways that obstruct the action of Vif could provide a new strategy for blocking viral replication. We demonstrate here that the host protein ASK1 (apoptosis signal-regulating kinase 1) interferes with the counteraction by Vif and revitalizes A3G-mediated viral restriction. ASK1 binds the BC-box of Vif, thereby disrupting the assembly of the Vif-ubiquitin ligase complex. Consequently, ASK1 stabilizes A3G and promotes its incorporation into viral particles, ultimately reducing viral infectivity. Furthermore, treatment with the antiretroviral drug AZT (zidovudine) induces ASK1 expression and restores the antiviral activity of A3G in HIV-1-infected cells. This study thus demonstrates a distinct function of ASK1 in restoring the host antiviral system that can be enhanced by AZT treatment.
Figures



Similar articles
-
Identification of a novel HIV-1 inhibitor targeting Vif-dependent degradation of human APOBEC3G protein.J Biol Chem. 2015 Apr 17;290(16):10504-17. doi: 10.1074/jbc.M114.626903. Epub 2015 Feb 27. J Biol Chem. 2015. PMID: 25724652 Free PMC article.
-
Induction of heat-shock protein 70 by prostaglandin A₁ inhibits HIV-1 Vif-mediated degradation of APOBEC3G.Antiviral Res. 2013 Sep;99(3):307-11. doi: 10.1016/j.antiviral.2013.06.017. Epub 2013 Jul 4. Antiviral Res. 2013. PMID: 23831493
-
Moloney leukemia virus 10 (MOV10) inhibits the degradation of APOBEC3G through interference with the Vif-mediated ubiquitin-proteasome pathway.Retrovirology. 2017 Dec 19;14(1):56. doi: 10.1186/s12977-017-0382-1. Retrovirology. 2017. PMID: 29258557 Free PMC article.
-
[Advances in the study of molecular mechanism of APOBEC3G anti-HIV-1].Yao Xue Xue Bao. 2008 Jul;43(7):678-82. Yao Xue Xue Bao. 2008. PMID: 18819469 Review. Chinese.
-
Various strategies for developing APOBEC3G protectors to circumvent human immunodeficiency virus type 1.Eur J Med Chem. 2023 Mar 15;250:115188. doi: 10.1016/j.ejmech.2023.115188. Epub 2023 Feb 6. Eur J Med Chem. 2023. PMID: 36773550 Review.
Cited by
-
Molecular dissection of HBV evasion from restriction factor tetherin: A new perspective for antiviral cell therapy.Oncotarget. 2015 Sep 8;6(26):21840-52. doi: 10.18632/oncotarget.4808. Oncotarget. 2015. PMID: 26334101 Free PMC article.
-
Structural basis of autoregulatory scaffolding by apoptosis signal-regulating kinase 1.Proc Natl Acad Sci U S A. 2017 Mar 14;114(11):E2096-E2105. doi: 10.1073/pnas.1620813114. Epub 2017 Feb 27. Proc Natl Acad Sci U S A. 2017. PMID: 28242696 Free PMC article.
-
Cyclin F/FBXO1 Interacts with HIV-1 Viral Infectivity Factor (Vif) and Restricts Progeny Virion Infectivity by Ubiquitination and Proteasomal Degradation of Vif Protein through SCFcyclin F E3 Ligase Machinery.J Biol Chem. 2017 Mar 31;292(13):5349-5363. doi: 10.1074/jbc.M116.765842. Epub 2017 Feb 9. J Biol Chem. 2017. PMID: 28184007 Free PMC article.
-
Multiple Inhibitory Factors Act in the Late Phase of HIV-1 Replication: a Systematic Review of the Literature.Microbiol Mol Biol Rev. 2018 Jan 10;82(1):e00051-17. doi: 10.1128/MMBR.00051-17. Print 2018 Mar. Microbiol Mol Biol Rev. 2018. PMID: 29321222 Free PMC article.
-
Assembly Dynamics and Stoichiometry of the Apoptosis Signal-regulating Kinase (ASK) Signalosome in Response to Electrophile Stress.Mol Cell Proteomics. 2016 Jun;15(6):1947-61. doi: 10.1074/mcp.M115.057364. Epub 2016 Mar 22. Mol Cell Proteomics. 2016. PMID: 27006476 Free PMC article.
References
-
- Medzhitov R. & Janeway C. Jr. Innate immunity. N. Engl. J. Med. 343, 338–344 (2000) . - PubMed
-
- Sheehy A. M., Gaddis N. C., Choi J. D. & Malim M. H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 (2002) . - PubMed
-
- Turelli P., Mangeat B., Jost S., Vianin S. & Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science 303, 1829 (2004) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

