Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model
- PMID: 25902068
- PMCID: PMC4406435
- DOI: 10.1371/journal.pgen.1005182
Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model
Abstract
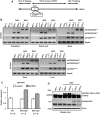
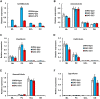
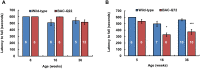
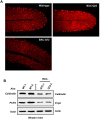
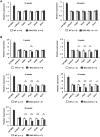
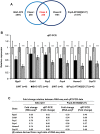
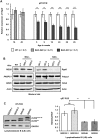
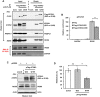
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant disorder with progressive degeneration of cerebellar Purkinje cells (PCs) and other neurons caused by expansion of a glutamine (Q) tract in the ATXN2 protein. We generated BAC transgenic lines in which the full-length human ATXN2 gene was transcribed using its endogenous regulatory machinery. Mice with the ATXN2 BAC transgene with an expanded CAG repeat (BAC-Q72) developed a progressive cellular and motor phenotype, whereas BAC mice expressing wild-type human ATXN2 (BAC-Q22) were indistinguishable from control mice. Expression analysis of laser-capture microdissected (LCM) fractions and regional expression confirmed that the BAC transgene was expressed in PCs and in other neuronal groups such as granule cells (GCs) and neurons in deep cerebellar nuclei as well as in spinal cord. Transcriptome analysis by deep RNA-sequencing revealed that BAC-Q72 mice had progressive changes in steady-state levels of specific mRNAs including Rgs8, one of the earliest down-regulated transcripts in the Pcp2-ATXN2[Q127] mouse line. Consistent with LCM analysis, transcriptome changes analyzed by deep RNA-sequencing were not restricted to PCs, but were also seen in transcripts enriched in GCs such as Neurod1. BAC-Q72, but not BAC-Q22 mice had reduced Rgs8 mRNA levels and even more severely reduced steady-state protein levels. Using RNA immunoprecipitation we showed that ATXN2 interacted selectively with RGS8 mRNA. This interaction was impaired when ATXN2 harbored an expanded polyglutamine. Mutant ATXN2 also reduced RGS8 expression in an in vitro coupled translation assay when compared with equal expression of wild-type ATXN2-Q22. Reduced abundance of Rgs8 in Pcp2-ATXN2[Q127] and BAC-Q72 mice supports our observations of a hyper-excitable mGluR1-ITPR1 signaling axis in SCA2, as RGS proteins are linked to attenuating mGluR1 signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Antisense oligonucleotide therapy for spinocerebellar ataxia type 2.Nature. 2017 Apr 20;544(7650):362-366. doi: 10.1038/nature22044. Epub 2017 Apr 12. Nature. 2017. PMID: 28405024 Free PMC article.
-
Spinocerebellar Ataxia Type 2.Adv Exp Med Biol. 2018;1049:175-195. doi: 10.1007/978-3-319-71779-1_8. Adv Exp Med Biol. 2018. PMID: 29427103 Review.
-
Generation of an Atxn2-CAG100 knock-in mouse reveals N-acetylaspartate production deficit due to early Nat8l dysregulation.Neurobiol Dis. 2019 Dec;132:104559. doi: 10.1016/j.nbd.2019.104559. Epub 2019 Jul 31. Neurobiol Dis. 2019. PMID: 31376479
-
Atxn2 Knockout and CAG42-Knock-in Cerebellum Shows Similarly Dysregulated Expression in Calcium Homeostasis Pathway.Cerebellum. 2017 Feb;16(1):68-81. doi: 10.1007/s12311-016-0762-4. Cerebellum. 2017. PMID: 26868665 Free PMC article.
-
Electrophysiological Studies Support Utility of Positive Modulators of SK Channels for the Treatment of Spinocerebellar Ataxia Type 2.Cerebellum. 2022 Oct;21(5):742-749. doi: 10.1007/s12311-021-01349-1. Epub 2022 Jan 3. Cerebellum. 2022. PMID: 34978024 Review.
Cited by
-
Degenerative ataxias, from genes to therapies: The 2015 Cotzias Lecture.Neurology. 2016 Jun 14;86(24):2284-90. doi: 10.1212/WNL.0000000000002777. Neurology. 2016. PMID: 27298447 Free PMC article. Review.
-
Slc9a6 mutation causes Purkinje cell loss and ataxia in the shaker rat.Hum Mol Genet. 2023 May 5;32(10):1647-1659. doi: 10.1093/hmg/ddad004. Hum Mol Genet. 2023. PMID: 36621975 Free PMC article.
-
Cerebellar Transcriptome Profiles of ATXN1 Transgenic Mice Reveal SCA1 Disease Progression and Protection Pathways.Neuron. 2016 Mar 16;89(6):1194-1207. doi: 10.1016/j.neuron.2016.02.011. Epub 2016 Mar 3. Neuron. 2016. PMID: 26948890 Free PMC article.
-
Gene co-expression network analysis for identifying modules and functionally enriched pathways in SCA2.Hum Mol Genet. 2017 Aug 15;26(16):3069-3080. doi: 10.1093/hmg/ddx191. Hum Mol Genet. 2017. PMID: 28525545 Free PMC article.
-
Antisense oligonucleotide therapy for spinocerebellar ataxia type 2.Nature. 2017 Apr 20;544(7650):362-366. doi: 10.1038/nature22044. Epub 2017 Apr 12. Nature. 2017. PMID: 28405024 Free PMC article.
References
-
- Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, et al. (1996) Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 14: 269–276. - PubMed
-
- Zoghbi HY (1995) Spinocerebellar ataxia type 1. Clin Neurosci 3: 5–11. - PubMed
-
- Pulst SM, Santos N, Wang D, Yang H, Huynh D, et al. (2005) Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset. Brain 128: 2297–2303. - PubMed
-
- Shibata H, Huynh DP, Pulst SM (2002) A novel protein with RNA-binding motifs interacts with ataxin-2. Hum Mol Genet 9: 1303–1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

