Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells
- PMID: 25902315
- PMCID: PMC4406474
- DOI: 10.1371/journal.pone.0125206
Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells
Abstract
Aim: To investigate RBC-NOS dependent NO signaling during in vivo RBC aging in health and disease.
Method: RBC from fifteen healthy volunteers (HC) and four patients with type 2 diabetes mellitus (DM) were separated in seven subpopulations by Percoll density gradient centrifugation.
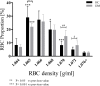
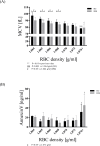
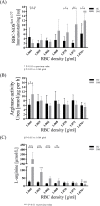
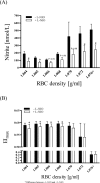
Results: The proportion of old RBC was significantly higher in DM compared to HC. In both groups, in vivo aging was marked by changes in RBC shape and decreased cell volume. RBC nitrite, as marker for NO, was higher in DM and increased in both HC and DM during aging. RBC deformability was lower in DM and significantly decreased in old compared to young RBC in both HC and DM. RBC-NOS Serine1177 phosphorylation, indicating enzyme activation, increased during aging in both HC and DM. Arginase I activity remained unchanged during aging in HC. In DM, arginase I activity was significantly higher in young RBC compared to HC but decreased during aging. In HC, concentration of L-arginine, the substrate of RBC-NOS and arginase I, significantly dropped from young to old RBC. In DM, L-arginine concentration was significantly higher in young RBC compared to HC and significantly decreased during aging. In blood from healthy subjects, RBC-NOS activation was additionally inhibited by N5-(1-iminoethyl)-L-Ornithine dihydrochloride which decreased RBC nitrite, and impaired RBC deformability of all but the oldest RBC subpopulation.
Conclusion: This study first-time showed highest RBC-NOS activation and NO production in old RBC, possibly to counteract the negative impact of cell shrinkage on RBC deformability. This was even more pronounced in DM. It is further suggested that highly produced NO only insufficiently affects cell function of old RBC maybe because of isolated RBC-NOS in old RBC thus decreasing NO bioavailability. Thus, increasing NO availability may improve RBC function and may extend cell life span in old RBC.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

