Examining kinesin processivity within a general gating framework
- PMID: 25902401
- PMCID: PMC4453223
- DOI: 10.7554/eLife.07403
Examining kinesin processivity within a general gating framework
Abstract
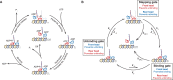
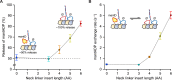
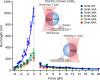
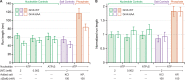
Kinesin-1 is a dimeric motor that transports cargo along microtubules, taking 8.2-nm steps in a hand-over-hand fashion. The ATP hydrolysis cycles of its two heads are maintained out of phase by a series of gating mechanisms, which lead to processive runs averaging ~1 μm. A key structural element for inter-head coordination is the neck linker (NL), which connects the heads to the stalk. To examine the role of the NL in regulating stepping, we investigated NL mutants of various lengths using single-molecule optical trapping and bulk fluorescence approaches in the context of a general framework for gating. Our results show that, although inter-head tension enhances motor velocity, it is crucial neither for inter-head coordination nor for rapid rear-head release. Furthermore, cysteine-light mutants do not produce wild-type motility under load. We conclude that kinesin-1 is primarily front-head gated, and that NL length is tuned to enhance unidirectional processivity and velocity.
Keywords: E. coli; biophysics; human; molecular motor; optical trap; single molecule; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



Comment in
-
Small steps and giant leaps.Elife. 2015 Jun 3;4:e08366. doi: 10.7554/eLife.08366. Elife. 2015. PMID: 26039082 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

