A dendritic-cell-stromal axis maintains immune responses in lymph nodes
- PMID: 25902483
- PMCID: PMC4591553
- DOI: 10.1016/j.immuni.2015.03.015
A dendritic-cell-stromal axis maintains immune responses in lymph nodes
Abstract
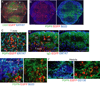
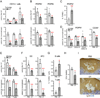
Within secondary lymphoid tissues, stromal reticular cells support lymphocyte function, and targeting reticular cells is a potential strategy for controlling pathogenic lymphocytes in disease. However, the mechanisms that regulate reticular cell function are not well understood. Here we found that during an immune response in lymph nodes, dendritic cells (DCs) maintain reticular cell survival in multiple compartments. DC-derived lymphotoxin beta receptor (LTβR) ligands were critical mediators, and LTβR signaling on reticular cells mediated cell survival by modulating podoplanin (PDPN). PDPN modulated integrin-mediated cell adhesion, which maintained cell survival. This DC-stromal axis maintained lymphocyte survival and the ongoing immune response. Our findings provide insight into the functions of DCs, LTβR, and PDPN and delineate a DC-stromal axis that can potentially be targeted in autoimmune or lymphoproliferative diseases.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


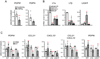

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

