Accessory NUMM (NDUFS6) subunit harbors a Zn-binding site and is essential for biogenesis of mitochondrial complex I
- PMID: 25902503
- PMCID: PMC4426432
- DOI: 10.1073/pnas.1424353112
Accessory NUMM (NDUFS6) subunit harbors a Zn-binding site and is essential for biogenesis of mitochondrial complex I
Abstract
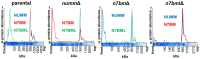
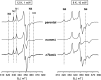
Mitochondrial proton-pumping NADH:ubiquinone oxidoreductase (respiratory complex I) comprises more than 40 polypeptides and contains eight canonical FeS clusters. The integration of subunits and insertion of cofactors into the nascent complex is a complicated multistep process that is aided by assembly factors. We show that the accessory NUMM subunit of complex I (human NDUFS6) harbors a Zn-binding site and resolve its position by X-ray crystallography. Chromosomal deletion of the NUMM gene or mutation of Zn-binding residues blocked a late step of complex I assembly. An accumulating assembly intermediate lacked accessory subunit N7BM (NDUFA12), whereas a paralog of this subunit, the assembly factor N7BML (NDUFAF2), was found firmly bound instead. EPR spectroscopic analysis and metal content determination after chromatographic purification of the assembly intermediate showed that NUMM is required for insertion or stabilization of FeS cluster N4.
Keywords: FeS cluster; NDUFA12; NDUFAF2; assembly; metal protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brandt U. Energy converting NADH:quinone oxidoreductase (complex I) Annu Rev Biochem. 2006;75:69–92. - PubMed
-
- Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013;82:551–575. - PubMed
-
- Hunte C, Zickermann V, Brandt U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science. 2010;329(5990):448–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

