Injury alters intrinsic functional connectivity within the primate spinal cord
- PMID: 25902510
- PMCID: PMC4434735
- DOI: 10.1073/pnas.1424106112
Injury alters intrinsic functional connectivity within the primate spinal cord
Abstract
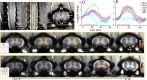
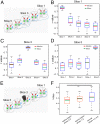
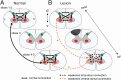
Recent demonstrations of correlated low-frequency MRI signal variations between subregions of the spinal cord at rest in humans, similar to those found in the brain, suggest that such resting-state functional connectivity constitutes a common feature of the intrinsic organization of the entire central nervous system. We report our detection of functional connectivity within the spinal cords of anesthetized squirrel monkeys at rest and show that the strength of connectivity within these networks is altered by the effects of injuries. By quantifying the low-frequency MRI signal correlations between different horns within spinal cord gray matter, we found distinct functional connectivity relationships between the different sensory and motor horns, a pattern that was similar to activation patterns evoked by nociceptive heat or tactile stimulation of digits. All horns within a single spinal segment were functionally connected, with the strongest connectivity occurring between ipsilateral dorsal and ventral horns. Each horn was strongly connected to the same horn on neighboring segments, but this connectivity reduced drastically along the spinal cord. Unilateral injury to the spinal cord significantly weakened the strength of the intrasegment horn-to-horn connectivity only on the injury side and in slices below the lesion. These findings suggest resting-state functional connectivity may be a useful biomarker of functional integrity in injured and recovering spinal cords.
Keywords: cervical spinal cord; hand; monkey; resting state fMRI; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. - PubMed
-
- Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

