Essential role of Orai1 store-operated calcium channels in lactation
- PMID: 25902527
- PMCID: PMC4426473
- DOI: 10.1073/pnas.1502264112
Essential role of Orai1 store-operated calcium channels in lactation
Abstract
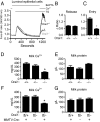
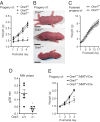
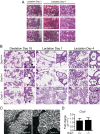
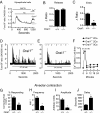
The nourishment of neonates by nursing is the defining characteristic of mammals. However, despite considerable research into the neural control of lactation, an understanding of the signaling mechanisms underlying the production and expulsion of milk by mammary epithelial cells during lactation remains largely unknown. Here we demonstrate that a store-operated Ca(2+) channel subunit, Orai1, is required for both optimal Ca(2+) transport into milk and for milk ejection. Using a novel, 3D imaging strategy, we visualized live oxytocin-induced alveolar unit contractions in the mammary gland, and we demonstrated that in this model milk is ejected by way of pulsatile contractions of these alveolar units. In mammary glands of Orai1 knockout mice, these contractions are infrequent and poorly coordinated. We reveal that oxytocin also induces a large transient release of stored Ca(2+) in mammary myoepithelial cells followed by slow, irregular Ca(2+) oscillations. These oscillations, and not the initial Ca(2+) transient, are mediated exclusively by Orai1 and are absolutely required for milk ejection and pup survival, an observation that redefines the signaling processes responsible for milk ejection. These findings clearly demonstrate that Ca(2+) is not just a substrate for nutritional enrichment in mammals but is also a master regulator of the spatiotemporal signaling events underpinning mammary alveolar unit contraction. Orai1-dependent Ca(2+) oscillations may represent a conserved language in myoepithelial cells of other secretory epithelia, such as sweat glands, potentially shedding light on other Orai1 channelopathies, including anhidrosis (an inability to sweat).
Keywords: calcium channels; calcium signaling; lactation; mammary gland; store-operated calcium entry.
Conflict of interest statement
Conflict of interest statement: S.F. is a cofounder of Calcimedica.
Figures
References
-
- Watson CJ, Khaled WT. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development. 2008;135(6):995–1003. - PubMed
-
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–725. - PubMed
-
- Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: Targets for breast cancer. Biochim Biophys Acta. 2006;1765(2):235–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

