Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis
- PMID: 25902530
- PMCID: PMC4426438
- DOI: 10.1073/pnas.1502400112
Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis
Abstract
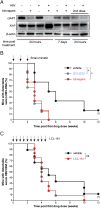
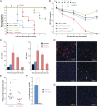
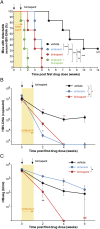
We have shown that cellular inhibitor of apoptosis proteins (cIAPs) impair clearance of hepatitis B virus (HBV) infection by preventing TNF-mediated killing/death of infected cells. A key question, with profound therapeutic implications, is whether this finding can be translated to the development of drugs that promote elimination of infected cells. Drug inhibitors of cIAPs were developed as cancer therapeutics to promote TNF-mediated tumor killing. These drugs are also known as Smac mimetics, because they mimic the action of the endogenous protein Smac/Diablo that antagonizes cIAP function. Here, we show using an immunocompetent mouse model of chronic HBV infection that birinapant and other Smac mimetics are able to rapidly reduce serum HBV DNA and serum HBV surface antigen, and they promote the elimination of hepatocytes containing HBV core antigen. The efficacy of Smac mimetics in treating HBV infection is dependent on their chemistry, host CD4(+) T cells, and TNF. Birinapant enhances the ability of entecavir, an antiviral nucleoside analog, to reduce viral DNA production in HBV-infected animals. These results indicate that birinapant and other Smac mimetics may have efficacy in treating HBV infection and perhaps, other intracellular infections.
Keywords: Smac mimetic; TNF; birinapant; cellular inhibitor of apoptosis proteins; hepatitis B virus.
Conflict of interest statement
Conflict of interest statement: The Walter and Eliza Hall Institute of Medical Research has a research license agreement with TetraLogic Pharmaceuticals Corporation, Inc., the manufacturer of the cellular inhibitor of apoptosis protein antagonist birinapant. TetraLogic Pharmaceuticals Corporation, Inc. has filed a patent cooperation treaty application on behalf of The Walter and Eliza Hall Institute of Medical Research. J.S. is on the scientific advisory board of and M.P. provides consultative advice to TetraLogic Pharmaceuticals Corporation, Inc. J.S. has options on a small number of shares in TetraLogic Pharmaceuticals Corporation, Inc. C.G.B. is employed by TetraLogic Pharmaceuticals Corporation, Inc.
Figures

References
-
- Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2013;31(1):118–121. - PubMed
-
- Lan JL, et al. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 2011;70(10):1719–1725. - PubMed
-
- Silke J. The regulation of TNF signalling: What a tangled web we weave. Curr Opin Immunol. 2011;23(5):620–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

