Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part I: Influenza life-cycle and currently available drugs
- PMID: 25902573
- PMCID: PMC4718311
Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part I: Influenza life-cycle and currently available drugs
Abstract
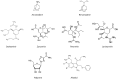
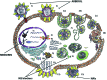
Influenza is a contagious respiratory acute viral disease characterized by a short incubation period, high fever and respiratory and systemic symptoms. The burden of influenza is very heavy. Indeed, the World Health Organization (WHO) estimates that annual epidemics affect 5-15% of the world's population, causing up to 4-5 million severe cases and from 250,000 to 500,000 deaths. In order to design anti-influenza molecules and compounds, it is important to understand the complex replication cycle of the influenza virus. Replication is achieved through various stages. First, the virus must engage the sialic acid receptors present on the free surface of the cells of the respiratory tract. The virus can then enter the cells by different routes (clathrin-mediated endocytosis or CME, caveolae-dependent endocytosis or CDE, clathrin-caveolae-independent endocytosis, or macropinocytosis). CME is the most usual pathway; the virus is internalized into an endosomal compartment, from which it must emerge in order to release its nucleic acid into the cytosol. The ribonucleoprotein must then reach the nucleus in order to begin the process of translation of its genes and to transcribe and replicate its nucleic acid. Subsequently, the RNA segments, surrounded by the nucleoproteins, must migrate to the cell membrane in order to enable viral assembly. Finally, the virus must be freed to invade other cells of the respiratory tract. All this is achieved through a synchronized action of molecules that perform multiple enzymatic and catalytic reactions, currently known only in part, and for which many inhibitory or competitive molecules have been studied. Some of these studies have led to the development of drugs that have been approved, such as Amantadine, Rimantadine, Oseltamivir, Zanamivir, Peramivir, Laninamivir, Ribavirin and Arbidol. This review focuses on the influenza life-cycle and on the currently available drugs, while potential antiviral compounds for the prevention and treatment of influenza are considered in the subsequent review.
Figures

References
-
- Schutten M, Baalen C, Zoeteweij P, et al. The influenza virus: disease, diagnostics, and treatment. MLO Med Lab Obs. 2013;45:38–40. - PubMed
-
- CDC , author. Seasonal Influenza: Flu Basics. Document available at: http://www.cdc.gov/flu/about/disease/index.htm. Accessed on 01 August 2014.
-
- Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6:114–124. - PubMed
-
- Short KR, Habets MN, Hermans PW, et al. Interactions between streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol. 2012;7:609–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
