Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: a randomized controlled trial
- PMID: 25902844
- PMCID: PMC4407555
- DOI: 10.1186/s12916-015-0315-6
Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: a randomized controlled trial
Abstract
Background: Ready-to-use therapeutic foods (RUTF) are lipid-based pastes widely used in the treatment of acute malnutrition. Current specifications for RUTF permit a high n-6 polyunsaturated fatty acid (PUFA) content and low n-3 PUFA, with no stipulated requirements for preformed long-chain n-3 PUFA. The objective of this study was to develop an RUTF with elevated short-chain n-3 PUFA and measure its impact, with and without fish oil supplementation, on children's PUFA status during treatment of severe acute malnutrition.
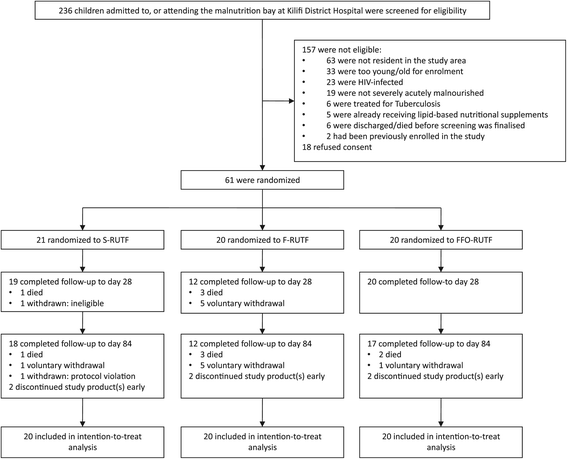
Methods: This randomized controlled trial in children with severe acute malnutrition in rural Kenya included 60 children aged 6 to 50 months who were randomized to receive i) RUTF with standard composition; ii) RUTF with elevated short chain n-3 PUFA; or iii) RUTF with elevated short chain n-3 PUFA plus fish oil capsules. Participants were followed-up for 3 months. The primary outcome was erythrocyte PUFA composition.
Results: Erythrocyte docosahexaenoic acid (DHA) content declined from baseline in the two arms not receiving fish oil. Erythrocyte long-chain n-3 PUFA content following treatment was significantly higher for participants in the arm receiving fish oil than for those in the arms receiving RUTF with elevated short chain n-3 PUFA or standard RUTF alone: 3 months after enrollment, DHA content was 6.3% (interquartile range 6.0-7.3), 4.5% (3.9-4.9), and 3.9% (2.4-5.7) of total erythrocyte fatty acids (P <0.001), respectively, while eicosapentaenoic acid (EPA) content was 2.0% (1.5-2.6), 0.7% (0.6-0.8), and 0.4% (0.3-0.5) (P <0.001). RUTF with elevated short chain n-3 PUFA and fish oil capsules were acceptable to participants and carers, and there were no significant differences in safety outcomes.
Conclusions: PUFA requirements of children with SAM are not met by current formulations of RUTF, or by an RUTF with elevated short-chain n-3 PUFA without additional preformed long-chain n-3 PUFA. Clinical and growth implications of revised formulations need to be addressed in large clinical trials.
Trial registration: Clinicaltrials.gov NCT01593969. Registered 4 May 2012.
Figures


Comment in
-
Balancing omega-6 and omega-3 fatty acids in ready-to-use therapeutic foods (RUTF).BMC Med. 2015 May 15;13:117. doi: 10.1186/s12916-015-0352-1. BMC Med. 2015. PMID: 25980919 Free PMC article.
References
-
- Briend A, Myatt M, Dent N, Brown R. Putting kwashiorkor on the map. CMAM Forum 2013. http://www.cmamforum.org/Pool/Resources/Putting-kwashiorkor-on-the-map-C....
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

