Localization of cystic fibrosis transmembrane conductance regulator signaling complexes in human salivary gland striated duct cells
- PMID: 25903037
- PMCID: PMC4425606
- DOI: 10.1111/eos.12184
Localization of cystic fibrosis transmembrane conductance regulator signaling complexes in human salivary gland striated duct cells
Abstract
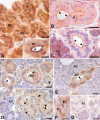
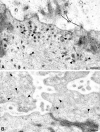
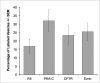
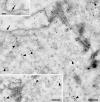
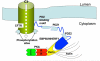
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic AMP-dependent protein kinase (PKA)-regulated Cl(-) channel, crucial for epithelial cell regulation of salt and water transport. Previous studies showed that ezrin, an actin binding and A-kinase anchoring protein (AKAP), facilitates association of PKA with CFTR. We used immunohistochemistry and immunogold transmission electron microscopy to localize CFTR, ezrin, and PKA type II regulatory (RII) and catalytic (C) subunits in striated duct cells of human parotid and submandibular glands. Immunohistochemistry localized the four proteins mainly to the apical membrane and the apical cytoplasm of striated duct cells. In acinar cells, ezrin localized to the luminal membrane, and PKA RII subunits were present in secretory granules, as previously described. Immunogold labeling showed that CFTR and PKA RII and C subunits were localized to the luminal membrane and associated with apical granules and vesicles of striated duct cells. Ezrin was present along the luminal membrane, on microvilli and along the junctional complexes between cells. Double labeling showed specific protein associations with apical granules and vesicles and along the luminal membrane. Ezrin, CFTR, and PKA RII and C subunits are co-localized in striated duct cells, suggesting the presence of signaling complexes that serve to regulate CFTR activity.
Keywords: immunohistochemistry; parotid gland; protein kinase A; submandibular gland; transmission electron microscopy.
© 2015 Eur J Oral Sci.
Figures



References
-
- WELSH MJ, BOAT TF, TSUI L-C, BEAUDET AL. Cystic Fibrosis. In: Seriven CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. McGraw Hill; New York, NY: 1995. pp. 3799–3876.
-
- PRANKE IM. SERMET-GAUDELUS I. Biosynthesis of cystic fibrosis transmembrane conductance regulator. Int J Biochem Cell Biol. 2014 http://dx.doi.org/10.1016/j.biocel.2014.03.020. - DOI - PubMed
-
- CANT N, POLLOCK N, FORD RC. CFTR structure and cystic fibrosis. Int J Biochem Cell Biol. 2014 http://dx.doi.org/10.1016/j.biocel.2014.02.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

