EPHB4 Protein Expression in Vascular Smooth Muscle Cells Regulates Their Contractility, and EPHB4 Deletion Leads to Hypotension in Mice
- PMID: 25903126
- PMCID: PMC4447992
- DOI: 10.1074/jbc.M114.621615
EPHB4 Protein Expression in Vascular Smooth Muscle Cells Regulates Their Contractility, and EPHB4 Deletion Leads to Hypotension in Mice
Abstract
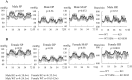
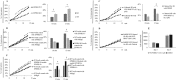
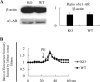
EPH kinases are the largest family of receptor tyrosine kinases, and their ligands, ephrins (EFNs), are also cell surface molecules. This work presents evidence that EPHB4 on vascular smooth muscle cells (VSMCs) is involved in blood pressure regulation. We generated gene KO mice with smooth muscle cell-specific deletion of EPHB4. Male KO mice, but not female KO mice, were hypotensive. VSMCs from male KO mice showed reduced contractility when compared with their WT counterparts. Signaling both from EFNBs to EPHB4 (forward signaling) and from EPHB4 to EFNB2 (reverse signaling) modulated VSMC contractility. At the molecular level, the absence of EPHB4 in VSMCs resulted in compromised signaling from Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) to myosin light chain kinase (MLCK) to myosin light chain, the last of which controls the contraction force of motor molecule myosin. Near the cell membrane, an adaptor protein GRIP1, which can associate with EFNB2, was found to be essential in mediating EPHB4-to-EFNB reverse signaling, which regulated VSMC contractility, based on siRNA gene knockdown studies. Our research indicates that EPHB4 plays an essential role in regulating small artery contractility and blood pressure.
Keywords: EPHB4 kinases; GRIP1; cell biology; gene knockout; hormone; hypertension; hypotension; sex hormones; vascular smooth muscle cells.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Eph Nomenclature Committee (1997) Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 90, 403–404 - PubMed
-
- Pasquale E. B. (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 - PubMed
-
- Wu J., Luo H. (2005) Recent advances on T-cell regulation by receptor tyrosine kinases. Curr. Opin. Hematol. 12, 292–297 - PubMed
-
- Flanagan J. G., Vanderhaeghen P. (1998) The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345 - PubMed
-
- Luo H., Charpentier T., Wang X., Qi S., Han B., Wu T., Terra R., Lamarre A., Wu J. (2011) Efnb1 and Efnb2 proteins regulate thymocyte development, peripheral T cell differentiation, and antiviral immune responses and are essential for interleukin-6 (IL-6) signaling. J. Biol. Chem. 286, 41135–41152 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

