Rac-mediated Stimulation of Phospholipase Cγ2 Amplifies B Cell Receptor-induced Calcium Signaling
- PMID: 25903139
- PMCID: PMC4498044
- DOI: 10.1074/jbc.M115.645739
Rac-mediated Stimulation of Phospholipase Cγ2 Amplifies B Cell Receptor-induced Calcium Signaling
Abstract
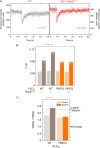
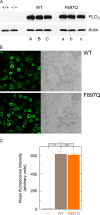
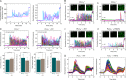
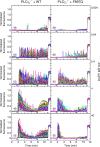
The Rho GTPase Rac is crucially involved in controlling multiple B cell functions, including those regulated by the B cell receptor (BCR) through increased cytosolic Ca(2+). The underlying molecular mechanisms and their relevance to the functions of intact B cells have thus far remained unknown. We have previously shown that the activity of phospholipase Cγ2 (PLCγ2), a key constituent of the BCR signalosome, is stimulated by activated Rac through direct protein-protein interaction. Here, we use a Rac-resistant mutant of PLCγ2 to functionally reconstitute cultured PLCγ2-deficient DT40 B cells and to examine the effects of the Rac-PLCγ2 interaction on BCR-mediated changes of intracellular Ca(2+) and regulation of Ca(2+)-regulated and nuclear-factor-of-activated-T-cell-regulated gene transcription at the level of single, intact B cells. The results show that the functional Rac-PLCγ2 interaction causes marked increases in the following: (i) sensitivity of B cells to BCR ligation; (ii) BCR-mediated Ca(2+) release from intracellular stores; (iii) Ca(2+) entry from the extracellular compartment; and (iv) nuclear translocation of the Ca(2+)-regulated nuclear factor of activated T cells. Hence, Rac-mediated stimulation of PLCγ2 activity serves to amplify B cell receptor-induced Ca(2+) signaling.
Keywords: B cell; Rac (Rac GTPase); calcium; lymphocyte; phospholipase C; signal amplification; signal transduction.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Kadamur G., Ross E. M. (2013) Mammalian phospholipase C. Annu. Rev. Physiol. 75, 127–154 - PubMed
-
- Tedder T. F. (2010) Innate and adaptive receptors interact to balance humoral immunity. J. Immunol. 184, 2231–2232 - PubMed
-
- de Gorter D. J., Beuling E. A., Kersseboom R., Middendorp S., van Gils J. M., Hendriks R. W., Pals S. T., Spaargaren M. (2007) Bruton's tyrosine kinase and phospholipase Cγ2 mediate chemokine-controlled B cell migration and homing. Immunity 26, 93–104 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

