A Survey of Marine Natural Compounds and Their Derivatives with Anti-cancer Activity Reported in 2012
- PMID: 25903364
- PMCID: PMC6272635
- DOI: 10.3390/molecules20047097
A Survey of Marine Natural Compounds and Their Derivatives with Anti-cancer Activity Reported in 2012
Abstract
Although considerable effort and progress has been made in the search for new anticancer drugs and treatments in the last several decades, cancer remains a major public health problem and one of the major causes of death worldwide. Many sources, including plants, animals, and minerals, are of interest in cancer research because of the possibility of identifying novel molecular therapeutics. Moreover, structure-activity-relationship (SAR) investigations have become a common way to develop naturally derived or semi-synthetic molecular analogues with improved efficacy and decreased toxicity. In 2012, approximately 138 molecules from marine sources, including isolated compounds and their associated analogues, were shown to be promising anticancer drugs. Among these, 62% are novel compounds. In this report, we review the marine compounds identified in 2012 that may serve as novel anticancer drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













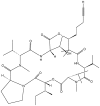
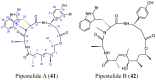




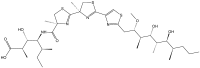
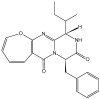

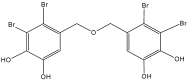
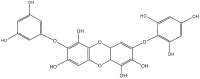


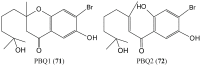


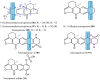

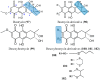

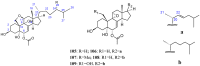

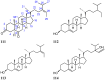

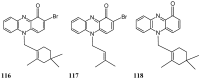



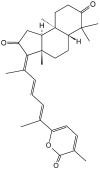



References
-
- IARC . Cancer Incidence and Mortality Worlwide. International Agency for Research on Cancer; Lyon, France: 2011.
-
- WHO . Global Status Report on Noncommunicable Diseases 2010. WHO; Geneva, Switzerland: 2011. pp. 11–15.
-
- WHO Cancer: Fact Sheet N°297. [(accessed on 8 October 2014)]. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/
-
- Sarfaraj H.M., Sheeba F., Saba A., Mohd S.K. Marine natural products: A lead for anticancer. Indian J. Geo-Mar. Sci. 2012;41:27–39.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

