Quantitative morphological changes in the interplacentomal wall of the gravid uterine horn of cattle during pregnancy
- PMID: 25903583
- PMCID: PMC4407553
- DOI: 10.1186/s12958-015-0030-3
Quantitative morphological changes in the interplacentomal wall of the gravid uterine horn of cattle during pregnancy
Abstract
Background: The interplacentomal wall of the gravid uterine horn in cattle is the subject of reports dealing mainly with specific aspects of early pregnancy or the peripartal period. Only a very limited number of early and descriptive studies includes the whole period of pregnancy. Thus, there is a gap concerning quantitative morphological data of the uterine wall during pregnancy. We hypothesized that the specific requirements of pregnancy are reflected by significant and characteristic morphologic changes.
Methods: Interplacentomal segments of the fetus-bearing horn of the uterus of 47 cows were collected at slaughter, assessed quantitatively by light microscopy, grouped into trimesters (trim), and data were analyzed statistically.
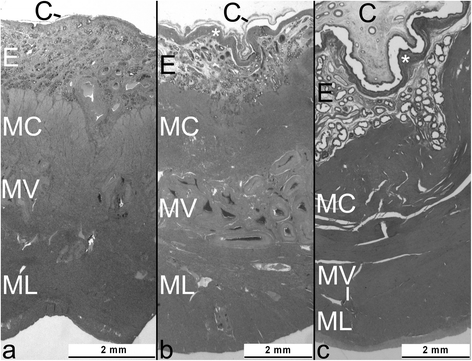
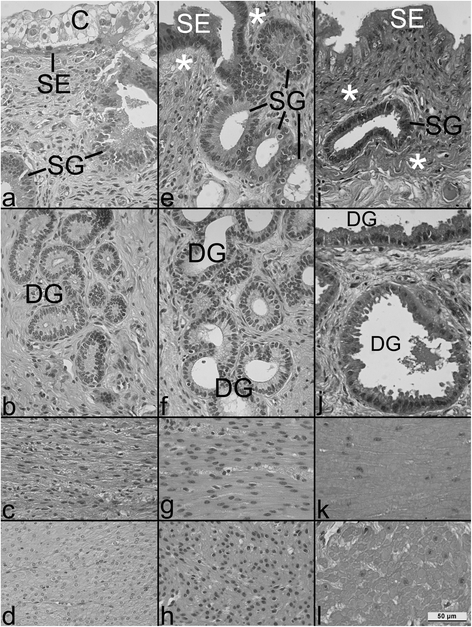
Results: During pregnancy there were significant increases (p<0.05) in the measured parameters: heights of the endometrial surface epithelium (31 increased to 46 and 46 μm, in the 1st, 2nd and 3rd trim, respectively), glandular epithelium (19.6 to 22.4 and 25.4 μm, respectively), diameters of glands (94 to 166 to 239 μm, respectively) and glandular lumina (56 to 122 to 188 μm, respectively). Volume density of the glandular epithelium did not change, while that of glandular lumina increased significantly (8 to 26 to 40% in the 1st, 2nd and 3rd trim, respectively) and of endometrial stroma decreased with ongoing pregnancy (67 to 46 to 37%; p<0.05). Diameters of myometrial smooth muscle cells (MSMC) (9.7 to 12.4 and 12.9 μm, respectively, for the 1st, 2nd and 3rd trim; p<0.05), and the volume fraction of myometrial stroma increased (6 to 10 to 13%; p<0.05), while decreases were observed in MSMC nuclear volume density (4.4 and 4.0 to 2.4%; p<0.05). The fraction of MSMC cytoplasm (89 to 85%) and the nucleus:cytoplasm ratio (0.05 to 0.03%) both decreased for the 1st vs. 3rd trim, respectively (p<0.05).
Conclusions: These results indicate that the interplacentomal wall of the gravid uterine horn is subjected to significant morphological changes during pregnancy, underlining the importance of endometrial surface epithelium and of gland hypertrophy for nourishment of the conceptus, of increased myometrial extracellular matrix for uterine tensile strength and of myometrial smooth muscle hypertrophy for expulsion of the fetus at term.
Figures
Similar articles
-
Pregnancy effects on distribution of progesterone receptors, oestrogen receptor alpha, glucocorticoid receptors, Ki-67 antigen and apoptosis in the bovine interplacentomal uterine wall and foetal membranes.Anim Reprod Sci. 2006 Jan;91(1-2):55-76. doi: 10.1016/j.anireprosci.2005.03.012. Anim Reprod Sci. 2006. PMID: 15885934
-
Immunohistochemical demonstration of cyclooxygenase-2 (COX-2) and prostaglandin receptors EP2 and FP expression in the bovine intercaruncular uterine wall around term.Anim Reprod Sci. 2008 Jul;106(3-4):241-54. doi: 10.1016/j.anireprosci.2007.04.016. Epub 2007 May 3. Anim Reprod Sci. 2008. PMID: 17574782
-
Immunocytochemical localization of estrogen and progestin receptors in the baboon (Papio anubis) uterus during implantation and pregnancy.Endocrinology. 1992 Apr;130(4):2343-53. doi: 10.1210/endo.130.4.1372241. Endocrinology. 1992. PMID: 1372241
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
-
Progesterone and placental hormone actions on the uterus: insights from domestic animals.Biol Reprod. 2004 Jul;71(1):2-10. doi: 10.1095/biolreprod.103.024133. Epub 2004 Feb 18. Biol Reprod. 2004. PMID: 14973264 Review.
Cited by
-
Exploring the Effects of Artemisia absinthium L. Essential Oil on Pseudopregnancy Model in Rats.Vet Med Sci. 2025 Sep;11(5):e70582. doi: 10.1002/vms3.70582. Vet Med Sci. 2025. PMID: 40844671 Free PMC article.
References
-
- Spencer TE, Gray CA. Sheep uterine gland knockout (UGKO) model. Methods Mol Med. 2006;121:85–94. - PubMed
-
- Ferrell CL. Maternal and fetal influences on uterine and conceptus development in the cow: I. Growth of tissues of the gravid uterus. J Anim Sci. 1991;69(5):1945–53. - PubMed
-
- Prior RL, Laster DB. Development of the bovine fetus. J Anim Sci. 1979;48(6):1546–53. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

