A new heart for a new head in vertebrate cardiopharyngeal evolution
- PMID: 25903628
- PMCID: PMC4851342
- DOI: 10.1038/nature14435
A new heart for a new head in vertebrate cardiopharyngeal evolution
Abstract
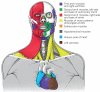
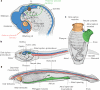
It has been more than 30 years since the publication of the new head hypothesis, which proposed that the vertebrate head is an evolutionary novelty resulting from the emergence of neural crest and cranial placodes. Neural crest generates the skull and associated connective tissues, whereas placodes produce sensory organs. However, neither crest nor placodes produce head muscles, which are a crucial component of the complex vertebrate head. We discuss emerging evidence for a surprising link between the evolution of head muscles and chambered hearts - both systems arise from a common pool of mesoderm progenitor cells within the cardiopharyngeal field of vertebrate embryos. We consider the origin of this field in non-vertebrate chordates and its evolution in vertebrates.
Figures
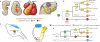

References
-
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–273. [This highly influential paper argued that the evolution of head structures derived from neural crest and cranial placodes had a crucial role in the transition to early vertebrates.] - PubMed
-
- Patthey C, Schlosser G, Shimeld SM. The evolutionary history of vertebrate cranial placodes — I: cell type evolution. Dev. Biol. 2014;389:82–97. - PubMed
-
- Northcutt RG. The new head hypothesis revisited. J. Exp. Zool. B Mol. Dev. Evol. 2005;304B:274–297. - PubMed
-
- Kuratani S. Evolution. A muscular perspective on vertebrate evolution. Science. 2013;341:139–140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

