Pei regimen: a therapeutic option in small cell lung cancer? A retrospective monoinstitutional analysis of 46 consecutive cases
- PMID: 25903963
- PMCID: PMC4427968
- DOI: 10.1186/s12967-015-0491-3
Pei regimen: a therapeutic option in small cell lung cancer? A retrospective monoinstitutional analysis of 46 consecutive cases
Abstract
Objectives: Combination chemotherapy is very active in small cell lung cancer (SCLC), although no improvement in overall survival (OS) has been done in the last 25 years, with Cisplatin-Etoposide (PE) still considered the world-wide standard, with an average median survival of about 7-8 months in patients with extended disease (ED). In 1995, a randomized trial of the Hoosier Group in 171 ED patients showed a significant advantage in overall survival in patients treated with PEI (Cisplatin, Etoposide and Ifosfamide), compared to PE. Despite that, PEI regimen has not become a commonly used regimen in SCLC.
Materials and methods: Here we present a retrospective analysis of 46 consecutive patients (30 males and 16 females) with SCLC that were treated at our Institution with PEI regimen: Cisplatin 20 mg/m2, Etoposide 75 mg/m2 and Ifosfamide 1200 mg/m2, day 1 to 4, every 3 weeks. Patients received a total of 219 cycles of chemotherapy, with a mean of 4,7 cycles per patient. Median age was 63 (range 59-70); performance status (PS) was 0 in 29 patients (63%), 1 in 13 patients (28%) and 2 in 4 patients (9%).
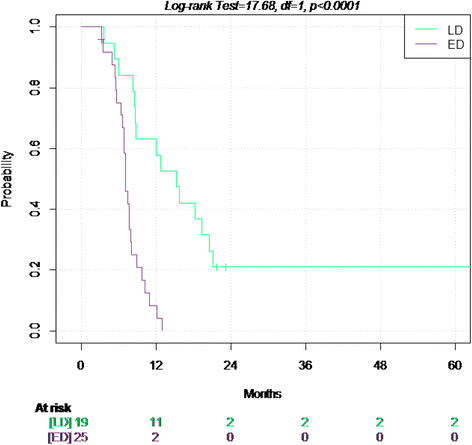
Results: In 19 limited disease (LD) patients partial response (PR) rate was 74%, and complete response (CR) was 16%. In 27 ED patients we observed 63% of PR and 26% of CR. Median time to progression (TTP) was 15.2 months in LD and 7.1 months in ED with median overall survival (OS) of 28.2 and 11.8 months, respectively. Toxicity was manageable, with a high dose intensity.
Conclusions: PEI regimen, in our opinion, may be a possible therapeutic option, with high activity and an acceptable toxicity profile.
Trial registration: ClinicalTrials.gov Identifier: NCT02324296 .
Institutional review board that approved the study: Institutional review board of Reggio Emilia, Azienda Ospedaliera S.Maria Nuova/IRCCS.
Figures
Similar articles
-
Experience of a German multicenter study group with ifosfamide in small cell lung cancer.Semin Oncol. 1989 Feb;16(1 Suppl 3):9-18. Semin Oncol. 1989. PMID: 2539648 Clinical Trial.
-
Cisplatin, Etoposide, and Bevacizumab Regimen Followed by Oral Etoposide and Bevacizumab Maintenance Treatment in Patients With Extensive-Stage Small Cell Lung Cancer: A Single-Institution Experience.Clin Lung Cancer. 2015 Nov;16(6):e229-34. doi: 10.1016/j.cllc.2015.05.005. Epub 2015 May 18. Clin Lung Cancer. 2015. PMID: 26072097
-
[Cisplatin etoposide versus cisplatin/etoposide/ifosfamide combination chemotherapy in small-cell lung cancer: a multicenter randomized controlled study. Hokkaido Study Group of Treatment for Small-Cell Lung Cancer].Gan To Kagaku Ryoho. 1991 Jun;18(7):1127-34. Gan To Kagaku Ryoho. 1991. PMID: 1647149 Clinical Trial. Japanese.
-
Etoposide plus ifosfamide plus cisplatin in the treatment of small cell lung cancer.Semin Oncol. 1998 Feb;25(1 Suppl 2):38-41. Semin Oncol. 1998. PMID: 9535210 Review.
-
Twenty-seven years of phase III trials for patients with extensive disease small-cell lung cancer: disappointing results.PLoS One. 2009 Nov 13;4(11):e7835. doi: 10.1371/journal.pone.0007835. PLoS One. 2009. PMID: 19915681 Free PMC article. Review.
Cited by
-
Real-world effectiveness and tolerability of small-cell lung cancer (SCLC) treatments: A systematic literature review (SLR).PLoS One. 2019 Jul 18;14(7):e0219622. doi: 10.1371/journal.pone.0219622. eCollection 2019. PLoS One. 2019. PMID: 31318909 Free PMC article.
References
-
- American Cancer Society . Cancer facts and figures 2012. 2012.
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years : analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials