Targeting breast cancer stem cells in triple-negative breast cancer using a combination of LBH589 and salinomycin
- PMID: 25904215
- PMCID: PMC4587767
- DOI: 10.1007/s10549-015-3376-5
Targeting breast cancer stem cells in triple-negative breast cancer using a combination of LBH589 and salinomycin
Abstract
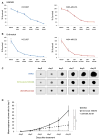
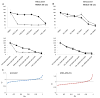
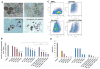
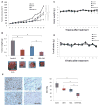
The aim of this study is to investigate the efficacy of combining a histone deacetylase inhibitor (LBH589) and a breast cancer stem cells (BCSC)-targeting agent (salinomycin) as a novel combination therapy for triple-negative breast cancer (TNBC). We performed in vitro studies using the TNBC cell lines to examine the combined effect. We used the mammosphere and ALDEFLUOR assays to estimate BCSC self-renewal capacity and distribution of BCSCs, respectively. Synergistic analysis was performed using CalcuSyn software. For in vivo studies, aldehyde dehydrogenase 1 ALDH1-positive cells were injected into non-obese diabetic/severe combined immunodeficiency gamma (NSG) mice. After tumor formation, mice were treated with LBH589, salinomycin, or in combination. In a second mouse model, HCC1937 cells were first treated with each treatment and then injected into NSG mice. For mechanistic analysis, immunohistochemistry and Western blot analysis were performed using cell and tumor samples. HCC1937 cells displayed BCSC properties including self-renewal capacity, an ALDH1-positive cell population, and the ability to form tumors. Treatment of HCC1937 cells with LBH589 and salinomycin had a potent synergistic effect inhibiting TNBC cell proliferation, ALDH1-positive cells, and mammosphere growth. In xenograft mouse models treated with LBH589 and salinomycin, the drug combination effectively and synergistically inhibited tumor growth of ALDH1-positive cells. The drug combination exerted its effects by inducing apoptosis, arresting the cell cycle, and regulating epithelial-mesenchymal transition (EMT). Combination of LBH589 and salinomycin has a synergistic inhibitory effect on TNBC BCSCs by inducing apoptosis, arresting the cell cycle, and regulating EMT; with no apparent associated severe toxicity. This drug combination could therefore offer a new targeted therapeutic strategy for TNBC and warrants further clinical study in patients with TNBC.
Conflict of interest statement
Figures


Similar articles
-
Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators.Breast Cancer Res Treat. 2014 Jun;145(3):593-604. doi: 10.1007/s10549-014-2979-6. Epub 2014 May 9. Breast Cancer Res Treat. 2014. PMID: 24810497 Free PMC article.
-
Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer.Oncol Rep. 2019 Jan;41(1):437-446. doi: 10.3892/or.2018.6805. Epub 2018 Oct 18. Oncol Rep. 2019. PMID: 30365081
-
Mevastatin blockade of autolysosome maturation stimulates LBH589-induced cell death in triple-negative breast cancer cells.Oncotarget. 2017 Mar 14;8(11):17833-17848. doi: 10.18632/oncotarget.14868. Oncotarget. 2017. PMID: 28147319 Free PMC article.
-
Targeting triple negative breast cancer with histone deacetylase inhibitors.Expert Opin Investig Drugs. 2017 Nov;26(11):1199-1206. doi: 10.1080/13543784.2017.1386172. Epub 2017 Oct 8. Expert Opin Investig Drugs. 2017. PMID: 28952409 Review.
-
STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review.J Exp Clin Cancer Res. 2019 May 14;38(1):195. doi: 10.1186/s13046-019-1206-z. J Exp Clin Cancer Res. 2019. PMID: 31088482 Free PMC article.
Cited by
-
Natural Products That Target Cancer Stem Cells.Anticancer Res. 2015 Nov;35(11):5773-88. Anticancer Res. 2015. PMID: 26503998 Free PMC article. Review.
-
ExoDS: a versatile exosome-based drug delivery platform to target cancer cells and cancer stem cells.Front Bioeng Biotechnol. 2024 Jun 5;12:1362681. doi: 10.3389/fbioe.2024.1362681. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38903193 Free PMC article.
-
Selectively Targeting Breast Cancer Stem Cells by 8-Quinolinol and Niclosamide.Int J Mol Sci. 2022 Oct 4;23(19):11760. doi: 10.3390/ijms231911760. Int J Mol Sci. 2022. PMID: 36233074 Free PMC article.
-
Preclinical and Clinical Trials of New Treatment Strategies Targeting Cancer Stem Cells in Subtypes of Breast Cancer.Cells. 2023 Feb 24;12(5):720. doi: 10.3390/cells12050720. Cells. 2023. PMID: 36899854 Free PMC article. Review.
-
Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line.Molecules. 2022 Jun 7;27(12):3661. doi: 10.3390/molecules27123661. Molecules. 2022. PMID: 35744786 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

