Flu-like and Other Systemic Drug Reactions Among Persons Receiving Weekly Rifapentine Plus Isoniazid or Daily Isoniazid for Treatment of Latent Tuberculosis Infection in the PREVENT Tuberculosis Study
- PMID: 25904367
- PMCID: PMC4560029
- DOI: 10.1093/cid/civ323
Flu-like and Other Systemic Drug Reactions Among Persons Receiving Weekly Rifapentine Plus Isoniazid or Daily Isoniazid for Treatment of Latent Tuberculosis Infection in the PREVENT Tuberculosis Study
Abstract
Background: Weekly rifapentine plus isoniazid for 3 months (3HP) is as effective as daily isoniazid for 9 months (9H) for latent tuberculosis infection in high-risk persons, but there have been reports of possible flu-like syndrome.
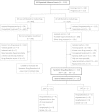
Methods: We identified clinically significant systemic drug reactions (SDR) and evaluated risk factors in patients who did not complete treatment in the PREVENT Tuberculosis study.
Results: Among 7552 persons who received ≥ 1 dose of study drug, 153 had a SDR: 138/3893 (3.5%) with 3HP vs 15/3659 (0.4%) with 9H (P < .001). In the 3HP arm, 87 (63%) had flu-like syndrome and 23 (17%) had cutaneous reactions; 13/3893 (0.3%) had severe reactions (6 were hypotensive) and 6 reported syncope. Symptoms occurred after a median of 3 doses, and 4 hours after the dose; median time to resolution was 24 hours. There were no deaths. In multivariate logistic regression analysis, factors independently associated with SDR included receipt of 3HP (adjusted odds ratio [aOR] 9.4; 95% confidence interval [CI], 5.5, 16.2), white non-Hispanic race/ethnicity (aOR 3.3; 95% CI, 2.3, 4.7), female sex (aOR 2.0; 95% CI, 1.4, 2.9), age ≥ 35 years (aOR 2.0; 95% CI, 1.4, 2.9), and lower body mass index (body mass index [BMI]; P = .009). In a separate multivariate analysis among persons who received 3HP, severe SDR were associated with white non-Hispanic race/ethnicity (aOR 5.4; 95% CI, 1.8, 16.3), and receipt of concomitant non-study medications (aOR 5.9; 95% CI, 1.3, 27.1).
Conclusions: SDR were more common with 3HP, and mostly flu-like. Persons of white race, female sex, older age, and lower BMI were at increased risk. Severe reactions were rare and associated with 3HP, concomitant medication, and white race. The underlying mechanism is unclear.
Clinical trials registration: NCT00023452.
Keywords: adverse drug reaction; flu-like syndrome; isoniazid; rifapentine; treatment of latent M. tuberculosis infection.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
References
-
- Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. - PubMed
-
- Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–3. - PubMed
-
- Bock NN, Sterling TR, Hamilton CD, et al. A prospective, randomized, double-blind study of the tolerability of rifapentine 600, 900, and 1,200 mg plus isoniazid in the continuation phase of tuberculosis treatment. Am J Respir Crit Care Med 2002; 165:1526–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

