Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s
- PMID: 25904376
- PMCID: PMC4542595
- DOI: 10.1093/cid/civ327
Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s
Erratum in
-
Erratum to: Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s.Clin Infect Dis. 2020 Dec 31;71(11):3020-3022. doi: 10.1093/cid/ciaa534. Clin Infect Dis. 2020. PMID: 33053151 Free PMC article. No abstract available.
Abstract
Background: It is unclear whether the integrase inhibitor raltegravir (RAL) reduces inflammation and immune activation compared with ritonavir-boosted protease inhibitors (PIs).
Methods: In a prospective, randomized, multicenter clinical trial that included 328 human immunodeficiency type 1 (HIV-1)-infected, treatment-naive participants were randomized to receive tenofovir disoproxil fumarate-emtricitabine (TDF/FTC) plus atazanavir/ritonavir (ATV/r), darunavir/ritonavir (DRV/r), or RAL. A total of 234 participants (71%) with HIV-1 RNA levels <50 copies/mL by week 24 were included. Plasma biomarkers of inflammation and coagulation that were analysed included high-sensitivity C-reactive protein, interleukin-6 (IL-6), GlycA, D-dimer, soluble CD14 (sCD14), sCD163, and sIL-2r; blood cellular markers included %CD38+DR+ of T-cell subsets and %CD14+CD16+ and%CD14(dim)CD16+ monocyte subsets. Changes from baseline were examined at earlier (24 or 48 weeks) and later (96 weeks) time points, with 95% confidence intervals on fold-change. Pairwise treatment groups were compared using Wilcoxon rank sum tests, with P values adjusted for false discovery rate control.
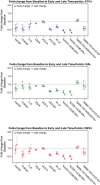
Results: Changes in biomarkers varied by regimen during the 96 weeks of follow-up as follows: hsCRP declined with ATV/r and RAL, IL-6 declined only with RAL, and GLycA decreased in all groups. D-dimer declined with ATV/r and DRV/r and was unchanged with RAL. Markers of T-cell activation and sCD163 (but not sCD14 and CD14-+CD16+) declined in all groups.
Conclusions: Despite some differences in specific markers of inflammation and immune activation between the antiretroviral therapy (ART) regimens, we found no consistent evidence that the reduction of inflammation and immune activation with ART initiation was different between RAL and PI-based regimens.
Clinical trials registration: NCT00811954 and NCT00851799.
Keywords: human immunodeficiency virus; immune activation; inflammation; integrase inhibitors; protease inhibitors.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069424/AI/NIAID NIH HHS/United States
- AI56933/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- K24 AI056933/AI/NIAID NIH HHS/United States
- K24 AI120834/AI/NIAID NIH HHS/United States
- AI69471/AI/NIAID NIH HHS/United States
- K08 AI108272/AI/NIAID NIH HHS/United States
- R01 HL095126/HL/NHLBI NIH HHS/United States
- R01 HL095132/HL/NHLBI NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- HL095132/HL/NHLBI NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI025915/AI/NIAID NIH HHS/United States
- K23 DA026308/DA/NIDA NIH HHS/United States
- AI 068636/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- HL095126/HL/NHLBI NIH HHS/United States
- AI068634/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

