Tolerance of Lung Allografts Achieved in Nonhuman Primates via Mixed Hematopoietic Chimerism
- PMID: 25904524
- PMCID: PMC4569127
- DOI: 10.1111/ajt.13274
Tolerance of Lung Allografts Achieved in Nonhuman Primates via Mixed Hematopoietic Chimerism
Abstract
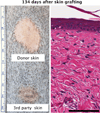
While the induction of transient mixed chimerism has tolerized MHC-mismatched renal grafts in nonhuman primates and patients, this approach has not been successful for more immunogenic organs. Here, we describe a modified delayed-tolerance-induction protocol resulting in three out of four monkeys achieving long-term lung allograft survival without ongoing immunosuppression. Two of the tolerant monkeys displayed stable mixed lymphoid chimerism, and the other showed transient chimerism. Serial biopsies and post-mortem specimens from the tolerant monkeys revealed no signs of chronic rejection. The tolerant recipients also exhibited T cell unresponsiveness and a lack of alloantibody. This is the first report of durable mixed chimerism and successful tolerance induction of MHC-mismatched lungs in primates.
Keywords: Animal models; basic (laboratory) research/science; lung transplantation/pulmonology; nonhuman primate; tolerance; translational research/science.
© Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures







References
-
- Sykes M, Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol Today. 1988;9:23–27. - PubMed
-
- Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–1098. - PubMed
-
- Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–1398. - PubMed
-
- Ochiai T, Benichou G, Cosimi AB, Kawai T. Induction of allograft tolerance in nonhuman primates and humans. Front Biosci. 2007;12:4248–4253. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

