Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier
- PMID: 25904743
- PMCID: PMC4435724
- DOI: 10.1126/scitranslmed.aaa4304
Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier
Erratum in
-
Erratum for the research article: "Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier" by H. M. Lazear, B. P. Daniels, A. K. Pinto, A. C. Huang, S. C. Vick, S. E. Doyle, M. Gale Jr., R. S. Klein, M. S. Diamond.Sci Transl Med. 2015 May 27;7(289):289er5. doi: 10.1126/scitranslmed.aac6108. Sci Transl Med. 2015. PMID: 26019215 No abstract available.
Abstract
Although interferon-λ [also known as type III interferon or interleukin-28 (IL-28)/IL-29] restricts infection by several viruses, its inhibitory mechanism has remained uncertain. We used recombinant interferon-λ and mice lacking the interferon-λ receptor (IFNLR1) to evaluate the effect of interferon-λ on infection with West Nile virus, an encephalitic flavivirus. Cell culture studies in mouse keratinocytes and dendritic cells showed no direct antiviral effect of exogenous interferon-λ, even though expression of interferon-stimulated genes was induced. We observed no differences in West Nile virus burden between wild-type and Ifnlr1(-/-) mice in the draining lymph nodes, spleen, or blood. We detected increased West Nile virus infection in the brain and spinal cord of Ifnlr1(-/-) mice, yet this was not associated with a direct antiviral effect in mouse neurons. Instead, we observed an increase in blood-brain barrier permeability in Ifnlr1(-/-) mice. Treatment of mice with pegylated interferon-λ2 resulted in decreased blood-brain barrier permeability, reduced West Nile virus infection in the brain without affecting viremia, and improved survival against lethal virus challenge. An in vitro model of the blood-brain barrier showed that interferon-λ signaling in mouse brain microvascular endothelial cells increased transendothelial electrical resistance, decreased virus movement across the barrier, and modulated tight junction protein localization in a protein synthesis- and signal transducer and activator of transcription 1 (STAT1)-independent manner. Our data establish an indirect antiviral function of interferon-λ in which noncanonical signaling through IFNLR1 tightens the blood-brain barrier and restricts viral neuroinvasion and pathogenesis.
Copyright © 2015, American Association for the Advancement of Science.
Figures
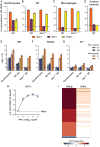

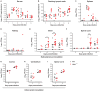

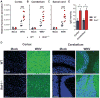
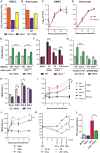

Comment in
-
Is the third interferon a charm?Sci Transl Med. 2015 Apr 22;7(284):284fs16. doi: 10.1126/scitranslmed.aaa2817. Epub 2015 Apr 22. Sci Transl Med. 2015. PMID: 25904738
References
-
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. - PubMed
-
- Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Günther S, Drosten C, Michiels T, Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

