Population responses in V1 encode different figures by response amplitude
- PMID: 25904787
- PMCID: PMC6605218
- DOI: 10.1523/JNEUROSCI.0971-14.2015
Population responses in V1 encode different figures by response amplitude
Abstract
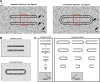
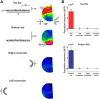
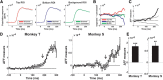
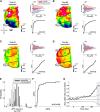
The visual system simultaneously segregates between several objects presented in a visual scene. The neural code for encoding different objects or figures is not well understood. To study this question, we trained two monkeys to discriminate whether two elongated bars are either separate, thus generating two different figures, or connected, thus generating a single figure. Using voltage-sensitive dyes, we imaged at high spatial and temporal resolution V1 population responses evoked by the two bars, while keeping their local attributes similar among the two conditions. In the separate condition, unlike the connected condition, the population response to one bar is enhanced, whereas the response to the other is simultaneously suppressed. The response to the background remained unchanged between the two conditions. This divergent pattern developed ∼200 ms poststimulus onset and could discriminate well between the separate and connected single trials. The stimulus separation saliency and behavioral report were highly correlated with the differential response to the bars. In addition, the proximity and/or the specific location of the connectors seemed to have only a weak effect on this late activity pattern, further supporting the involvement of top-down influences. Additional neural codes were less informative about the separate and connected conditions, with much less consistency and discriminability compared with a response amplitude code. We suggest that V1 is involved in the encoding of each figure by different neuronal response amplitude, which can mediate their segregation and perception.
Keywords: amplitude code; behaving monkeys; figure ground; object representation; primary visual cortex; voltage-sensitive dye imaging.
Copyright © 2015 the authors 0270-6474/15/356335-15$15.00/0.
Figures




Comment in
-
The Neural Representation of Multiple Objects in the Primate Visual System.J Neurosci. 2015 Sep 16;35(37):12612-4. doi: 10.1523/JNEUROSCI.2527-15.2015. J Neurosci. 2015. PMID: 26377452 Free PMC article. No abstract available.
Similar articles
-
Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys.J Neurophysiol. 2002 Dec;88(6):3421-38. doi: 10.1152/jn.00194.2002. J Neurophysiol. 2002. PMID: 12466458
-
Population response to natural images in the primary visual cortex encodes local stimulus attributes and perceptual processing.J Neurosci. 2012 Oct 3;32(40):13971-86. doi: 10.1523/JNEUROSCI.1596-12.2012. J Neurosci. 2012. PMID: 23035105 Free PMC article.
-
Population response to contextual influences in the primary visual cortex.Cereb Cortex. 2010 Jun;20(6):1293-304. doi: 10.1093/cercor/bhp191. Epub 2009 Sep 16. Cereb Cortex. 2010. PMID: 19759123
-
High response reliability of neurons in primary visual cortex (V1) of alert, trained monkeys.Cereb Cortex. 2006 Jun;16(6):888-95. doi: 10.1093/cercor/bhj032. Epub 2005 Sep 8. Cereb Cortex. 2006. PMID: 16151177
-
Dynamic binding of visual features by neuronal/stimulus synchrony.Appl Human Sci. 1998 May;17(3):97-108. doi: 10.2114/jpa.17.97. Appl Human Sci. 1998. PMID: 9682519 Review.
Cited by
-
Wide-field imaging in behaving mice as a tool to study cognitive function.Neurophotonics. 2024 Jul;11(3):033404. doi: 10.1117/1.NPh.11.3.033404. Epub 2024 Feb 19. Neurophotonics. 2024. PMID: 38384657 Free PMC article. Review.
-
Gap junctional coupling between retinal amacrine and ganglion cells underlies coherent activity integral to global object perception.Proc Natl Acad Sci U S A. 2017 Nov 28;114(48):E10484-E10493. doi: 10.1073/pnas.1708261114. Epub 2017 Nov 13. Proc Natl Acad Sci U S A. 2017. PMID: 29133423 Free PMC article.
-
Contextual modulation revealed by optical imaging exhibits figural asymmetry in macaque V1 and V2.Eye Brain. 2017 Apr 11;9:1-12. doi: 10.2147/EB.S105609. eCollection 2017. Eye Brain. 2017. PMID: 28761385 Free PMC article.
-
Catching the voltage gradient-asymmetric boost of cortical spread generates motion signals across visual cortex: a brief review with special thanks to Amiram Grinvald.Neurophotonics. 2017 Jul;4(3):031206. doi: 10.1117/1.NPh.4.3.031206. Epub 2017 Feb 10. Neurophotonics. 2017. PMID: 28217713 Free PMC article. Review.
-
Advanced Circuit and Cellular Imaging Methods in Nonhuman Primates.J Neurosci. 2019 Oct 16;39(42):8267-8274. doi: 10.1523/JNEUROSCI.1168-19.2019. J Neurosci. 2019. PMID: 31619496 Free PMC article. Review.
References
-
- Arieli A, Shoham D, Hildesheim R, Grinvald A. Coherent spatiotemporal patterns of ongoing activity revealed by real-time optical imaging coupled with single-unit recording in the cat visual cortex. J Neurophysiol. 1995;73:2072–2093. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
