Costimulation of AMPA and metabotropic glutamate receptors underlies phospholipase C activation by glutamate in hippocampus
- PMID: 25904792
- PMCID: PMC6605221
- DOI: 10.1523/JNEUROSCI.4208-14.2015
Costimulation of AMPA and metabotropic glutamate receptors underlies phospholipase C activation by glutamate in hippocampus
Abstract
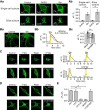
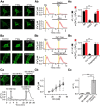
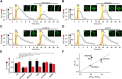
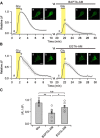
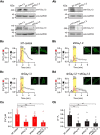
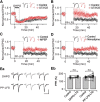
Glutamate, a major neurotransmitter in the brain, activates ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs, respectively). The two types of glutamate receptors interact with each other, as exemplified by the modulation of iGluRs by mGluRs. However, the other way of interaction (i.e., modulation of mGluRs by iGluRs) has not received much attention. In this study, we found that group I mGluR-specific agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) alone is not sufficient to activate phospholipase C (PLC) in rat hippocampus, while glutamate robustly activates PLC. These results suggested that additional mechanisms provided by iGluRs are involved in group I mGluR-mediated PLC activation. A series of experiments demonstrated that glutamate-induced PLC activation is mediated by mGluR5 and is facilitated by local Ca(2+) signals that are induced by AMPA-mediated depolarization and L-type Ca(2+) channel activation. Finally, we found that PLC and L-type Ca(2+) channels are involved in hippocampal mGluR-dependent long-term depression (mGluR-LTD) induced by paired-pulse low-frequency stimulation, but not in DHPG-induced chemical LTD. Together, we propose that AMPA receptors initiate Ca(2+) influx via the L-type Ca(2+) channels that facilitate mGluR5-PLC signaling cascades, which underlie mGluR-LTD in rat hippocampus.
Keywords: Cav1.2; Cav1.3; confocal imaging; electrophysiology; local calcium; mGluR-LTD.
Copyright © 2015 the authors 0270-6474/15/356401-12$15.00/0.
Figures
References
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
