Are striatal tyrosine hydroxylase interneurons dopaminergic?
- PMID: 25904808
- PMCID: PMC4405564
- DOI: 10.1523/JNEUROSCI.0195-15.2015
Are striatal tyrosine hydroxylase interneurons dopaminergic?
Abstract
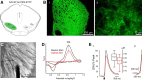
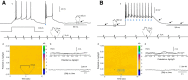
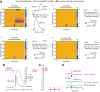
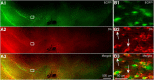
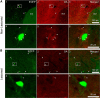
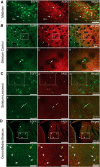
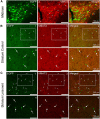
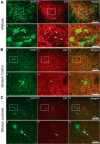
Striatal GABAergic interneurons that express the gene for tyrosine hydroxylase (TH) have been identified previously by several methods. Although generally assumed to be dopaminergic, possibly serving as a compensatory source of dopamine (DA) in Parkinson's disease, this assumption has never been tested directly. In TH-Cre mice whose nigrostriatal pathway had been eliminated unilaterally with 6-hydroxydopamine, we injected a Cre-dependent virus coding for channelrhodopsin-2 and enhanced yellow fluorescent protein unilaterally into the unlesioned midbrain or bilaterally into the striatum. Fast-scan cyclic voltammetry in striatal slices revealed that both optical and electrical stimulation readily elicited DA release in control striata but not from contralateral striata when nigrostriatal neurons were transduced. In contrast, neither optical nor electrical stimulation could elicit striatal DA release in either the control or lesioned striata when the virus was injected directly into the striatum transducing only striatal TH interneurons. This demonstrates that striatal TH interneurons do not release DA. Fluorescence immunocytochemistry in enhanced green fluorescent protein (EGFP)-TH mice revealed colocalization of DA, l-amino acid decarboxylase, the DA transporter, and vesicular monoamine transporter-2 with EGFP in midbrain dopaminergic neurons but not in any of the striatal EGFP-TH interneurons. Optogenetic activation of striatal EGFP-TH interneurons produced strong GABAergic inhibition in all spiny neurons tested. These results indicate that striatal TH interneurons are not dopaminergic but rather are a type of GABAergic interneuron that expresses TH but none of the other enzymes or transporters necessary to operate as dopaminergic neurons and exert widespread GABAergic inhibition onto direct and indirect spiny neurons.
Keywords: GABA; dopamine; interneuron; neostriatum; optogenetics; voltammetry.
Copyright © 2015 the authors 0270-6474/15/356584-16$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
