Glycolysis and the significance of lactate in traumatic brain injury
- PMID: 25904838
- PMCID: PMC4389375
- DOI: 10.3389/fnins.2015.00112
Glycolysis and the significance of lactate in traumatic brain injury
Abstract
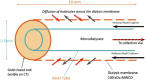
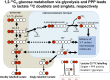
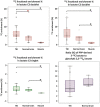
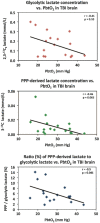
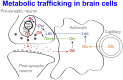
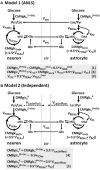
In traumatic brain injury (TBI) patients, elevation of the brain extracellular lactate concentration and the lactate/pyruvate ratio are well-recognized, and are associated statistically with unfavorable clinical outcome. Brain extracellular lactate was conventionally regarded as a waste product of glucose, when glucose is metabolized via glycolysis (Embden-Meyerhof-Parnas pathway) to pyruvate, followed by conversion to lactate by the action of lactate dehydrogenase, and export of lactate into the extracellular fluid. In TBI, glycolytic lactate is ascribed to hypoxia or mitochondrial dysfunction, although the precise nature of the latter is incompletely understood. Seemingly in contrast to lactate's association with unfavorable outcome is a growing body of evidence that lactate can be beneficial. The idea that the brain can utilize lactate by feeding into the tricarboxylic acid (TCA) cycle of neurons, first published two decades ago, has become known as the astrocyte-neuron lactate shuttle hypothesis. Direct evidence of brain utilization of lactate was first obtained 5 years ago in a cerebral microdialysis study in TBI patients, where administration of (13)C-labeled lactate via the microdialysis catheter and simultaneous collection of the emerging microdialysates, with (13)C NMR analysis, revealed (13)C labeling in glutamine consistent with lactate utilization via the TCA cycle. This suggests that where neurons are too damaged to utilize the lactate produced from glucose by astrocytes, i.e., uncoupling of neuronal and glial metabolism, high extracellular levels of lactate would accumulate, explaining the association between high lactate and poor outcome. Recently, an intravenous exogenous lactate supplementation study in TBI patients revealed evidence for a beneficial effect judged by surrogate endpoints. Here we review the current state of knowledge about glycolysis and lactate in TBI, how it can be measured in patients, and whether it can be modulated to achieve better clinical outcome.
Keywords: cerebral energy metabolism; glucose; glycolysis; lactate; microdialysis; pyruvate; traumatic brain injury (human).
Figures




References
-
- Bak L. K., Walls A. B., Schousboe A., Ring A., Sonnewald U., Waagepetersen H. S. (2009). Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J. Neurochem. 109 (Suppl. 1), 87–93. 10.1111/j.1471-4159.2009.05943.x - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

