Retinal Pigment Epithelial Cell Line Suppression of Phagolysosome Activation
- PMID: 25905107
- PMCID: PMC4403791
Retinal Pigment Epithelial Cell Line Suppression of Phagolysosome Activation
Abstract
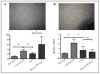
The eye is an immune privileged tissue with multiple mechanisms of immunosuppression to protect the light gathering tissues from the damage of inflammation. One of theses mechanisms involves retinal pigment epithelial cell suppression of phagosome activation in macrophages. The objective of this work is to determine if the human RPE cell line ARPE-19 is capable of suppressing the activation of the phagolysosome in macrophages in a manner similar to primary RPE. The conditioned media of RPE eyecups, sub-confluent, just confluent cultures, or established confluent cultures of human ARPE-19 cells were generated. These condition media were used to treat macrophages phagocytizing pHrodo bioparticles. After 24 hours incubation the macrophages were imaged by fluorescent microscopy, and fluorescence was measured. The fluorescent intensity is proportional to the amount of bioparticles phagocytized and are in an activated phagolysosome. The conditioned media of in situ mouse RPE eyecups significantly suppressed the activation of phagolysosome. The conditioned media from cultures of human ARPE-19 cells, grown to sub-confluence (50%) or grown to confluence had no effect on phagolysosome activation. In contrast, the conditioned media from established confluent cultures significantly suppressed phagolysosome activation. The neuropeptides alpha-MSH and NPY were depleted from the conditioned media of established confluent ARPE-19 cell cultures. This depleted conditioned media had diminished suppression of phagolysosome activation while promoting macrophage cell death. In addition, the condition media from cultures of ARPE-19 monolayers wounded with a bisecting scrape was diminished in suppressing phagolysosome activation. This technical report suggests that like primary RPE monolayers, established confluent cultures of ARPE-19 cells produce soluble factors that suppress the activation of macrophages, and can be used to study the molecular mechanisms of retinal immunobiology. In addition, the results further demonstrate the importance of an intact monolayer of RPE cells to modulate immune cell activity within the eye.
Keywords: Alpha-Melanocyte Stimulating Hormone; Immune Privilege; Macrophages; Neuroimmunomodulation; Neuropeptide Y; Phagocytosis.
Figures

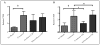

References
-
- Zamiri P, Masli S, Kitaichi N, Taylor A, Streilein J. Thrombospondin plays a vital role in the immune privilege of the eye. Investigative Ophthalmology & Visual Science. 2005;46:908–919. - PubMed
-
- Zamiri P, Masli S, Streilein JW, Taylor AW. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Investigative Ophthalmology & Visual Science. 2006;47:3912–3918. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
